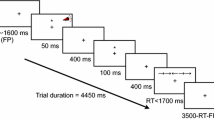
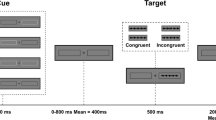
Abstract
This paper reports a series of experiments that were carried out in order to study the attentional system. Three networks make up this system, and each of them specializes in particular processes. The executive control network specializes in control processes, such as conflict resolution or detection of errors; the orienting network directs the processing system to the source of input and enhances its processing; the alerting network prepares the system for a fast response by maintaining an adequate level of activation in the cognitive system. Recently, Fan and collaborators [J Cogn Neurosci 14(3):340–347, 2002] designed a task to measure the efficiency of each network. We modified Fan’s task to test the influences among the networks. We found that the executive control network is inhibited by the alerting network, whereas the orienting network raises the efficiency of the executive control network (Experiment 1). We also found that the alerting network influences the orienting network by speeding up its time course function (Experiment 2). Results were replicated in a third experiment, proving the effects to be stable over time, participants and experimental context, and to be potentially important as a tool for neuropsychological assessment.




Similar content being viewed by others
Notes
Even though the orienting network is not exclusively concerned with visual information, but with input coming from any sense, for the sake of simplicity we only mention the studies concerning visual orienting since it is the most relevant to our tasks. See Spence and Driver (2005) for a review on crossmodal spatial orienting or Correa et al. (2004) for temporal orienting.
We used this nomenclature instead of the usual “valid/invalid” one to emphasize the absence of contingence between the location of the cue and that of the target.
The same criteria were used for all the experiments.
The results of the interactions including all the levels of the variables were: Visual Cue × Congruency: F (4,92)=6.31; p<0.001; Auditory Signal × Congruency: F (2,46)=2.60; p=0.08; Auditory Signal × Visual Cue: F (2,46)=39.26; p<0.0001.
This is easily explained by arguing that visual cues already produce some alerting, thus reducing the net effect produced by an auditory cue (Fernandez-Duque and Posner 1997).
The results of the interactions, including all the levels of the variables, were: Visual Cue × Congruency: F (2,94)=17.69; p<0.0001; Auditory Signal × Congruency does not change since the neutral level of the congruency variable was eliminated from the design; Auditory Signal × Visual Cue: F (2,94)=20.92; p<0.0001; SOA × Auditory Signal × Visual Cue: F (2,94)=1.45; p<0.24; Auditory Signal × Visual Cue (SOA 100): F (2,94)=9.6; p<0.0005; Auditory Signal × Visual Cue (SOA500): F (2,94)=9.47; p<0.0005.
Again all the main effects, as well as the interactions, pointed in the same direction as the previous findings. Mean RT and error rates per condition can be found in Table 1. Main effects: Auditory Signal: F (1,24)=29.66; p<0.0001; Visual Cue: F (2,48)=50.69; p<0.0001 and Congruency: F (1,24)=129.74; p<0.0001. Interactions: Visual Cue × Congruency: F (1,24)=5.94; p<0.05; Auditory Signal × Congruency: F (1,24)=8.84; p<0.01 and Auditory Signal × Visual Cue: F (1,24)=17.36; p<0.0005. See Callejas et al. (2004) for an extended report of a similar study.
Inhibition of return was not found in our studies. When a task is complex enough (such as our difficult discrimination task) IOR is not observed unless SOAs much longer than ours are used (Lupiáñez et al. 1997)
References
Allport A (1993) Attention and control: have we been asking the wrong question? A critical review of the last twenty-five years. In: Meyers DE, Kornblum S (eds) Attention and performance XIV. MIT Press, Cambridge, MA
Berger A, Posner MI (2000) Pathologies of brain attentional networks. Neurosci Biobehav Rev 24(1):3–5
Callejas A, Lupiáñez J, Tudela P (2004) The three attentional networks: on their independence and interactions. Brain Cogn 54:225–227
Casey BJ, Thomas KM, Welsh RF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA (2000) Dissociation of response conflict, attentional selection and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA 97(15):8728–8733
Cheal M, Chastain G (2002) Timing of facilitatory and inhibitory effects of visual attention. Visual Cogn 9:969–1002
Clark CR, Geffen GM, Geffen LB (1989) Catecholamines and covert orientation of attention in humans. Neuropsychologia 27(2):131–139
Cohen RM, Semple WE, Gross M, Holcomb HJ, Dowling SM, Nordahl TE (1988) Functional localization of sustained attention. Neuropsychiatry Neuropsychol Behav Neurol 1:3–20
Correa A, Lupiáñez J, Tudela P, Milliken B (2004) Endogenous temporal orienting of attention in detection and discrimination tasks. Percept Psychophys 66(2):264–278
Duncan J, Owen AM (2000) Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483
Eimer M (2000) The time course of spatial orienting elicited by central and peripheral cues: evidence from event-related brain potentials. Biol Psychol 53:253–258
Eriksen BA, Eriksen CW (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16(1):143–149
Fan J, Wu Y, Fossella JA, Posner MI (2001) Assessing the heritability of attentional networks. BMC Neurosci (electronic resource) 2:14–20
Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002) Testing the efficiency and independence of the attentional networks. J Cogn Neurosci 14(3):340–347
Fan J, McCandliss BD, Flombaum JI, Thomas KM, Posner MI (2003) Cognitive and brain consequences of conflict. Neuroimage 18:42–57
Fernandez-Duque D, Posner MI (1997) Relating the mechanisms of orienting and alerting. Neurosychologia 35(4):477–486
Fossella J, Posner MI, Fan J, Swanson JM, Pfaff DW (2002) Attentional phenotypes for the analysis of higher mental function. Sci World J 2:217–223
Funes MJ, Lupiáñez J (2003) La teoría atencional de Posner: una tarea para medir las funciones atencionales de orientación, alerta y control cognitivo y la interacción entre ellas. Psicothema 15(2):260–266
Funes MJ, Lupiáñez J, Milliken B (2005) Opposite effects of endogenous and exogenous spatial cues on the spatial stroop effect. J Exp Psychol Human (submitted)
Jones EG (1985) The thalamus. Plenum, New York
LaBerge D (2000) Networks of attention. In: Gazzaniga MS (ed) The new cognitive neurosciences, 2nd edn. MIT Press, Cambridge, MA
Lupiáñez J, Milán EG, Tornay F, Madrid E, Tudela P (1997) Does IOR occur in discrimination tasks? Yes, it does, but later. Percept Psychophys 59:1241–1254
Marrocco RT, Davidson MC (1999) Neurochemistry of attention. In: Parasuraman J (ed) The attentive brain. MIT Press, Cambridge, MA
Milliken B, Lupiáñez J, Roberts M, Stevanovski B (2003) Orienting in space and time: joint contributions to exogenous spatial cuing effects. Psychon Bull Rev 10:877–883
Morrison JH, Foote SL (1986) Noradrenergic and serotoninergic innervation of cortical, thalamic and tectal visual structures in Old and New World monkeys. J Comp Neurol 243(1):117–138
Müller HJ, Findlay JM (1988) The effect of visual attention on peripheral discrimination thresholds in single and multiple element displays. Acta Psychol 69(2):129–155
Müller HJ, Rabbitt PMA (1989) Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. J Exp Psychol Hum Percept Perform 15:315–330
Norman DA, Shallice T (1986) Attention to action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D (eds) Consciousness and self-regulation. Plenum, New York
Posner MI (1978) Chronometric explorations of mind. Erlbaum, Hillsdale, NJ
Posner MI (1980) Orienting of attention. Q J Exp Psychol 32:3–25
Posner MI (1994) Attention: the mechanisms of consciousness. Proc Natl Acad Sci USA 97:7398–7403
Posner MI, Boies SJ (1971) Components of attention. Psychol Rev 78(5):391–408
Posner MI, Cohen Y (1984) Components of visual orienting. In: Bouma H, Bouwhuis DG (eds) Attention and performance X. Erlbaum, Hillsdale, NJ, pp 531–556
Posner MI, Cohen A (1987) Isolating attentional systems: a cognitive-anatomical analysis. Psychobiology 15(2):107–121
Posner MI, DiGirolamo FJ (1998) Executive attention: conflict, target deterction and cognitive control. In: Parasuraman R (ed) The attentive brain. MIT Press, Cambridge, MA
Posner MI, Fan J (2005) Attention as an organ system. In: Pomerantz J (ed) Neurobiology of perception and communication: from synapse to society. The IVth de Lange conference. Cambridge University Press, Cambridge, UK (in press)
Posner MI, Petersen SE (1990) The attention system of the human brain. Annu Rev Neurosci 13:25–42
Posner MI, Raichle ME (1994) Images of mind. Scientific American Library, New York
Rizzolatti G, Fadiga L, Fogassi L, Gallese V (2002) From mirror neurons to imitation: Facts and speculations. In: Meltzoff A, Prinz W (eds) The imitative mind. Oxford University Press, New York
Robertson IH, Mattingley JB, Rorden C, Driver J (1998) Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature 395:169–172
Sapir A, Rafal R, Henik A (2002) Attending to the thalamus: inhibition of return and nasal-temporal asymmetry in the pulvinar. Neuroreport 13(5):693–697
Schneider W, Eschman A, Zuccolotto A (2002) E-Prime user’s guide. Psychology Software Tools Inc., Pittsburgh, PA
Spence C, Driver J (eds) (2004) Crossmodal space and crossmodal attention. Oxford University Press, Oxford
Sternberg S (1969) The discovery of processing stages: extensions of Donder’s method. Acta Psychol 30:276–315
Sturm W, Willmes K (2001) On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 14:S76–S84
Thiel CM, Zilles K, Fink GR (2004) Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. Neuroimage 21:318–328
Acknowledgments
We would like to thank Ray Klein and two anonymous reviewers for their helpful comments on earlier versions of the manuscript. This research was financially supported by the Spanish Ministerio de Educación, Cultura y Deporte with a predoctoral grant (FPU, AP2001-3181) to the first author, and by the Spanish Ministerio de Ciencia y Tecnología with research grants to the second and fourth authors (BSO2002-04308-C02-02 and BSO2000-1411-CO2, respectively).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Callejas, A., Lupiàñez, J., Funes, M.J. et al. Modulations among the alerting, orienting and executive control networks. Exp Brain Res 167, 27–37 (2005). https://doi.org/10.1007/s00221-005-2365-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-2365-z