Abstract
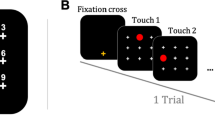
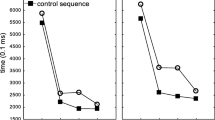
Motor sequence learning is a process whereby a series of elementary movements is re-coded into an efficient representation for the entire sequence. Here we show that human subjects learn a visuomotor sequence by spontaneously chunking the elementary movements, while each chunk acts as a single memory unit. The subjects learned to press a sequence of 10 sets of two buttons through trial and error. By examining the temporal patterns with which subjects performed a visuomotor sequence, we found that the subjects performed the 10 sets as several clusters of sets, which were separated by long time gaps. While the overall performance time decreased by repeating the same sequence, the clusters became clearer and more consistent. The cluster pattern was uncorrelated with the distance of hand movements and was different across subjects who learned the same sequence. We then split a learned sequence into three segments, while preserving or destroying the clusters in the learned sequence, and shuffled the segments. The performance on the shuffled sequence was more accurate and quicker when the clusters in the original sequence were preserved than when they were destroyed. The results suggest that each cluster is processed as a single memory unit, a chunk, and is necessary for efficient sequence processing. A learned visuomotor sequence is hierarchically represented as chunks that contain several elementary movements. We also found that the temporal patterns of sequence performance transferred from the nondominant to dominant hand, but not vice versa. This may suggest a role of the dominant hemisphere in storage of learned chunks. Together with our previous unit-recording and imaging studies that used the same learning paradigm, we predict specific roles of the dominant parietal area, basal ganglia, and presupplementary motor area in the chunking.







Similar content being viewed by others
References
Bapi RS, Doya K, Harner AM (2000) Evidence for effector independent and dependent representations and their differential time course of acquisition during motor sequence learning. Exp Brain Res 132:149–162
Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M (1998) The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 121:253–264
Cohen A, Ivry RI, Keele SW (1990) Attention and structure in sequence learning. J Exp Psychol Learn Mem Cognit 16:17–30
Curran T, Keele SW (1993) Attentional and nonattentional forms of sequence learning. J Exp Psychol Learn Mem Cognit 19:189–202
Dallal NL, Meck WH (1990) Hierarchical structures: chunking by food type facilitates spatial memory. J Exp Psychol Anim Behav Process 16:69–84
Ericsson KA, Chase WG, Faloon S (1980) Acquisition of a memory skill. Science 208:1181–1182
Fountain SB (1990) Rule abstraction, item memory, and chunking in rat serial-pattern tracking. J Exp Psychol Anim Behav Process 16:96–105
Gordon PC, Meyer DE (1984) Perceptual-motor processing of phonetic features in speech. J Exp Psychol Hum Percept Perform 10:153–178
Graybiel AM (1998) The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem 70:119–136
Harrington DL, Haaland KY (1991a) Hemispheric specialisation for motor sequencing: abnormalities in levels of programming. Neuropsychologia 29:147–163
Harrington DL, Haaland KY (1991b) Sequencing in Parkinson's disease. Abnormalities in programming and controlling movement. Brain 114:99–115
Harrington DL, Haaland KY (1992) Motor sequencing with left hemisphere damage: are some cognitive deficits specific to limb apraxia? Brain 115:857–874
Hikosaka O, Rand MK, Miyachi S, Miyashita K (1995) Learning of sequential movements in the monkey: process of learning and retention of memory. J Neurophysiol 74: 1652–1661
Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B (1996) Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol 76:617–621
Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K (1999a) Parallel neural networks for learning sequential procedures. Trends Neurosci 22:464–471
Hikosaka O, Sakai K, Nakahara H, Lu X, Miyachi S, Nakamura K, Rand MK (1999b) Neural mechanisms for learning of sequential procedures. In: Gazzaniga MS (ed) The new cognitive neurosciences. MIT Press, Cambridge, MA, pp 553–572
Hikosaka O, Nakamura K, Sakai K, Nakahara H (2002a) Central mechanisms of motor skill learning. Curr Opin Neurobiol 12:217–222
Hikosaka O, Rand MK, Nakamura K, Miyachi S, Kitaguchi K, Sakai K, Lu X, Shimo Y (2002b) Long-term retention of motor skill in macaque monkeys and humans. Exp Brain Res 147:494–504
Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM (1999) Building neural representations of habits. Science 286:1745–1749
Keele SW, Jennings PJ (1992) Attention in the representation of sequence: experiment and theory. Hum Mov Sci 11:125–138
Kennerley SW, Sakai K, Rushworth MFS (2002) The pre-SMA and sequential organization of actions: a TMS study. Abstr Soc Neurosci 163.8
Knowlton BJ, Mangels JA, Squire LR (1996) A neostriatal habit learning system in humans. Science 273:1399–1402
Koch I, Hoffmann J (2000) Patterns, chunks, and hierarchies in serial reaction-time tasks. Psychol Res 63:22–35
Lu X, Hikosaka O, Miyachi S (1998) Role of monkey cerebellar nuclei in skill for sequential movement. J Neurophysiol 79:2245–2254
Macuda T, Roberts WA (1995) Further evidence for hierarchical chunking in rat spatial memory. J Exp Psychol Anim Behav Process 21:20–32
Miller GA (1956) The magic number seven, plus or minus two: Some limits on our capacity for processing information. Psychol Rev 63:81–97
Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK (1997) Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res 115:1-5
Nakahara H, Doya K, Hikosaka O (2001) Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences: a computational approach. J Cognit Neurosci 13:626–647
Nakamura K, Sakai K, Hikosaka O (1998) Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol 80:2671–2687
Nakamura K, Sakai K, Hikosaka O (1999) Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol 82:1063–1068
Nissen MJ, Bullemer P (1987) Attentional requirements of learning: evidence from performance measures. Cognit Psychol 19:1-32
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Povel DJ, Collard R (1982) Structural factors in patterned finger tapping. Acta Psychologia 52:107–123
Rand MK, Hikosaka O, Miyachi S, Lu X, Miyashita K (1998) Characteristics of a long-term procedural skill in the monkey. Exp Brain Res 118:293–297
Rand MK, Hikosaka O, Miyachi S, Lu X, Nakamura K, Kitaguchi K, Shimo Y (2000) Characteristics of sequential movements during early learning period in monkeys. Exp Brain Res, 131:293–304
Restle F, Burnside BL (1972) Tracking of serial patterns. J Exp Psychol 95:299–307
Rushworth MFS, Nixon PD, Wade DT, Renowden S, Passingham RE (1998) The left hemisphere and the selection of learned actions. Neuropsychologia 36:11–24
Rosenbaum DA, Kenny SB, Derr MA (1983) Hierarchical control of rapid movement sequences. J Exp Psychol Hum Percept Perform 9:86–102
Sadato N, Campbell G, Ibanez V, Deiber M, Hallett M (1996) Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16:2691–2700
Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Putz B (1998) Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci 18:1827–1840
Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B (1999) Presupplementary motor area activation during sequence learning reflects visuomotor association. J Neurosci 19: RC1–5
Shima K, Mushiake H, Saito N, Tanji J (1996) Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA 93:8694–8698
Stadler MA (1989) On learning complex procedural knowledge. J Exp Psychol Learn Mem Cognit 15:1061–1069
Stadler MA (1993) Implicit serial learning: questions inspired by Hebb (1961) Mem Cognit 21:819–827
Terrace HS (1987) Chunking by a pigeon in a serial learning task. Nature 325:149–151
Terrace HS (1991) Chunking during serial learning by a pigeon. I. Basic evidence. J Exp Psychol Anim Behav Process 17:81–93
Terrace HS, Chen S (1991a) Chunking during serial learning by a pigeon. II. Integrity of a chunk on a new list. J Exp Psychol Anim Behav Process 17:94–106
Terrace HS, Chen S (1991b) Chunking during serial learning by a pigeon. III. What are the necessary conditions for establishing a chunk? J Exp Psychol Anim Behav Process 17:107–118
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakai, K., Kitaguchi, K. & Hikosaka, O. Chunking during human visuomotor sequence learning. Exp Brain Res 152, 229–242 (2003). https://doi.org/10.1007/s00221-003-1548-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1548-8