Abstract
Rationale
Among opioid-treated chronic pain patients, response inhibition deficits in emotional contexts may contribute to opioid misuse.
Objectives
Using high-frequency heart rate variability (HF-HRV) to index-impaired response inhibition, we examined associations between opioid misuse and response inhibition in emotional and neutral contexts in a sample of opioid-treated chronic pain patients.
Method
Chronic pain patients taking opioid analgesics (N = 97) for ≥ 90 days completed an Emotional Go/NoGo task that presented an inhibitory control challenge in the context of neutral, opioid, negative affective, and positive affective background images while HF-HRV was computed. Opioid misuse and craving were assessed. Using a validated cut-point on the Current Opioid Misuse Measure, participants were classified as opioid misusers or non-misusers. Opioid misuse was examined as a predictor of behavioral and HF-HRV metrics of response inhibition.
Results
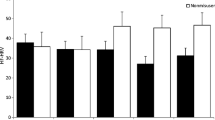
Negative affective and opioid images elicited more errors of commission (p = .002, η2partial = .16) and slowed reaction times (p = .045, η2partial = .09) compared to neutral and positive affective images, respectively. Though no between-group behavioral differences were observed on the task, opioid misusers exhibited significantly blunted phasic HF-HRV during the task relative to non-misusers (p = .027, η2partial = .11). HF-HRV during the task was significantly inversely associated with opioid craving. It was not clear whether these autonomic findings reflected a durable phenotypic difference between groups or between-group differences in opioid dosing and withdrawal.
Conclusion
Reduced parasympathetic regulation during inhibitory control challenge may indicate heightened opioid misuse risk among opioid-treated chronic pain patients.


Similar content being viewed by others
References
Albert J, Lopez-Martin S, Carretie L (2011) Emotional context modulates response inhibition: neural and behavioral data. Neuroimage 49:914–921
Appelhans BM, Luecken LJ (2006) Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol 10:229–240
Baldacchino A, Balfour DJK, Passetti F, Humphris G, Matthews K (2012) Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev 36:2056–2068
Baldacchino A, Balfour DJK, Matthews K (2015) Impulsivity and opioid drugs: differential effects of heroin, methadone and prescribed analgesic medication. Psychol Med 45:1167–1179
Baldo BA (2016) Prefrontal cortical opioids and dysregulated motivation: a network hypothesis. Trends Neurosci 39:366–377
Benarroch EE (1993) The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68:988–1001
Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufman PG, Malik M et al (1997) Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34:623–648
Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL (2014) Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev 34:563–579
Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN (2007) Development and validation of the current opioid misuse measure. Pain 130:144–156
Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA (2015) The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 162:276–286
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the Brief Pain Inventory. Annals, Academy of Medicine, Singapore
Di Simplicio M, Costoloni G, Western D, Hanson B, Taggart P, Harmer CJ (2012) Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychol Med 42(8):1775
Electrophysiology Task Force of the European Society Cardiologists (1996) Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065
Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM (2004) Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry 55:531–537
Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L (2008) Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett 438:322–326
Garland EL, Carter K, Ropes K, Howard MO (2012) Thought suppression, impaired regulation of urges, and Addiction-Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. Biol Psychol 89:87–93
Garland EL, Froeliger B, Howard M (2013a) Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Front Psychiatry 4:173
Garland EL, Froeliger BE, Passik SD, Howard MO (2013b) Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. J Behav Med 36:611–620
Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO (2013c) The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev 37:2597–2607
Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO (2014) Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol 82:448–459
Garland EL, Froeliger B, Howard MO (2015) Allostatic dysregulation of natural reward processing in prescription opioid misuse: autonomic and attentional evidence. Biol Psychol 105:124–129
Garland EL, Bryan CJ, Nakamura Y, Froeliger B, Howard MO (2016) Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology 234(4):621–629
Garland EL, Bryan MA, Priddy SE, Riquino MR, Froeliger B, Howard MO (2019) Effects of mindfulness-oriented recovery enhancement versus social support on negative affective interference during inhibitory control among opioid-treated chronic pain patients: a pilot mechanistic study. Ann Behav Med 53(10):865–876
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669
Hansen AL, Johnsen BH, Thayer JF (2003) Vagal influence on working memory and attention. Int J Psychophysiol 48:263–274
Holzman JB, Bridgett DJ (2017) Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev 74:233–255
Kendall SE, Sjøgren P, Pimenta CA, Højsted J, Kurita GP et al (2010) The cognitive effects of opioids in chronic non-cancer pain. Pain 150:225–230
Krypotos AM, Jahfari S, van Ast VA, Kindt M, Forstmann BU (2011) Individual differences in heart rate variability predict the degree of slowing during response inhibition and initiation in the presence of emotional stimuli. Front Psychol 2:278
Lang P, Bradley M, Cuthbert B (1997) International affective picture system (IAPS): technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention
Meltzer EC, Rybin D, Saitz R, Samet JH, Schwartz SL, Butler SF, Liebschutz JM (2011) Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). PAIN® 152(2):397–402
Mena JD, Selleck RA, Baldo BA (2013) Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci 33:18540–18552
Moeller SJ, Bederson L, Alia-Klein N, Goldstein RZ (2016) Neuroscience of inhibition for addiction medicine: from prediction of initiation to prediction of relapse. Prog Brain Res 223:165–188
Morgan TJ, Morgenstern J, Blanchard KA, Labouvie E, Bux DA (2004) Development of the OCDS-Revised: a measure of alcohol and drug urges with outpatient substance abuse clients. Psychol Addict Behav 18(4):316
Roos LE, Knight E, Beauchamp KG, Berkman ET, Faraday K, Hyslop K, Fisher PA (2017) Acute stress impairs inhibitory control based on individual differences in parasympathetic nervous system activity. Biol Psychol 125:58–63
Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM (2007) Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Arch Clin Neuropsychol 22:151–160
Segerstrom SC, Nes LS (2007) Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci 18:275–281
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:34–57
Smith JL, Mattick RP, Jamadar SD, Iredale JM (2014) Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend 145:1–33
Thayer JF, Lane RD (2000) A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61:201–216
Thayer JF, Lane RD (2009) Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 33:81–88
Thayer JF, Åhs F, Fredrikson M, Sollers JJ III, Wager TD (2012) A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 36:747–756
Tiffany ST (1990) A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 97:147–168
Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN (2015) Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156:569–576
Zacny JP (1995) A review of the effects of opioids on psychomotor and cognitive functioning in humans. Exp Clin Psychopharmacol 3:432–466
Zhou H, Dai Z, Hua L, Jiang H, Tian S, Han Y, Lin P, Wang H, Lu Q, Yao Z (2020) Decreased task-related HRV is associated with inhibitory dysfunction through functional inter-region connectivity of PFC in major depressive disorder. Front Psychiatry 10:989
Acknowledgments
This study was supported by NIDA grant R03DA032517 awarded to E.L.G. E.L.G. was supported by NIDA grant R01DA042033 during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garland, E.L., Howard, M.O. Prescription opioid misusers exhibit blunted parasympathetic regulation during inhibitory control challenge. Psychopharmacology 238, 765–774 (2021). https://doi.org/10.1007/s00213-020-05729-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05729-z