Abstract
Background
Rodent models highlight the key role of μ-opioid receptor (MOR) signaling in palatable food consumption. In humans, however, the effects of MOR stimulation on eating and food liking remain unclear.
Objectives
Here, we tested sweet pleasantness experience in humans following MOR drug manipulations. We hypothesized that behaviors regulated by the endogenous MOR system would be enhanced by MOR agonism and decreased by antagonism. In line with rodent findings, we expected the strongest drug effects for the sweetest (high-calorie) sucrose stimuli. As very sweet stimuli are considered aversive by many people (called sweet dislikers), we also assessed whether MOR manipulations affect pleasantness ratings of sucrose-water stimuli differently depending on subjective and objective value.
Methods
In a bidirectional psychopharmacological cross-over study, 49 healthy men underwent a sweet taste paradigm following double-blind administration of the MOR agonist morphine, placebo, and the opioid antagonist naltrexone.
Results
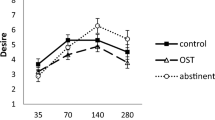
As hypothesized, MOR stimulation with morphine increased pleasantness of the sweetest of five sucrose solutions, without enhancing pleasantness of the lower-sucrose solutions. For opioid antagonism, an opposite pattern was observed for the sweetest drink only. The observed drug effects on pleasantness of the sweetest drink did not differ between sweet likers and dislikers.
Conclusions
The bidirectional effect of agonist and antagonist treatment aligns with rodent findings showing that MOR manipulations most strongly affect the highest-calorie foods. We speculate that the MOR system promotes survival in part by increasing concordance between the objective (caloric) and subjective (hedonic) value of food stimuli, so that feeding behavior becomes more focused on the richest food available.






Similar content being viewed by others
References
Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D (2008) An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the combined pharmacotherapies and behavioral interventions for alcohol dependence (COMBINE) study. Arch Gen Psychiatry 65:135–144
Arbisi P, Billington C, Levine A (1999) The effect of naltrexone on taste detection and recognition threshold. Appetite 32:241–249
Asao K, Miller J, Arcori L, Lumeng JC, Han-Markey T, Herman WH (2015) Patterns of sweet taste liking: a pilot study. Nutrients 7:7298–7311
Bellisle F (2010) Ingestive behaviours and sugar. Comportement alimentaire et sucre 4:511–513
Berman AH, Bergman H, Palmstierna T, Schlyter F (2005) Evaluation of the drug use disorders identification test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res 11:22–31
Berridge KC (2000) Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neuroscience &. Biobehav Rev 24:173–198
Berridge KC (2009) Wanting and liking: observations from the neuroscience and psychology laboratory. Inquiry 52:378–398
Berridge KC, Kringelbach ML (2008) Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology 199:457–480
Berthoud HR, Zheng H (2012) Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol Behav 107:527–532
Bertino M, Beauchamp GK, Engelman K (1991) Naltrexone, an opioid blocker, alters taste perception and nutrient intake in humans. Am J Phys Regul Integr Comp Phys 261:R59–R63
Chelnokova O, Laeng B, Eikemo M, Riegels J, Løseth G, Maurud H, Willoch F, Leknes S (2014) Rewards of beauty: the opioid system mediates social motivation in humans. Mol Psychiatry 19:746–747
Chidambaran V, Mavi J, Esslinger H, Pilipenko V, Martin LJ, Zhang K, Sadhasivam S (2015) Association of OPRM1 A118G variant with risk of morphine-induced respiratory depression following spine fusion in adolescents. Pharmacogenomics J 15:255–262
Cleary J, Weldon DT, O'Hare E, Billington C, Levine AS (1996) Naloxone effects on sucrose-motivated behavior. Psychopharmacology 126:110–114
Cousineau D (2005) Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology 1:42–45
DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC (2012) Enkephalin surges in dorsal neostriatum as a signal to eat. Curr Biol 22:1918–1924
Dlugos AM, Hamidovic A, Hodgkinson C, Shen PH, Goldman D, Palmer AA, De Wit H (2011) OPRM1 gene variants modulate amphetamine-induced euphoria in humans. Genes Brain Behav 10:199–209
Doyle TG, Berridge KC, Gosnell BA (1993) Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav 46:745–749
Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA (1992) Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav 51:371–379
Evans KR, Vaccarino FJ (1990) Amphetamine-and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev 14:9–22
Franke P, Wang T, Nöthen MM, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W (2001) Nonreplication of association between μ-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. Am J Med Genet 105:114–119
Garbutt JC, Osborne M, Gallop R, Barkenbus J, Grace K, Cody M, Flannery B, Kampov-Polevoy AB (2009) Treatment: sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol Alcohol 44:293–300
Giovannoni G, Van Schalkwyk J, Fritz VU, Lees AJ (1999) Bradykinesia akinesia incoordination test (BRAIN TEST): an objective computerised assessment of upper limb motor function. J Neurol Neurosurg Psychiatry 67:624–629
Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS (1993) Naloxone's anorectic effect is dependant upon the relative palatability of food. Pharmacol Biochem Behav 46:917–921
Gosnell BA, Mitra A, Avant RA, Anker JJ, Carroll ME, Levine AS (2010) Operant responding for sucrose by rats bred for high or low saccharin consumption. Physiol Behav 99:529–533
Gueorguieva R, Krystal JH (2004) Move over anova: progress in analyzing repeated-measures data andits reflection in papers published in the archives of general psychiatry. Arch Gen Psychiatry 61:310–317
Hetherington MM, Vervaet N, Blass E, Rolls BJ (1991) Failure of naltrexone to affect the pleasantness or intake of food. Pharmacol Biochem Behav 40:185–190
Janowsky DS, Pucilowski O, Buyinza M (2003) Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiatr Res 37:35–41
Kampov-Polevoy A, Lange L, Bobashev G, Eggleston B, Root T, Garbutt JC (2014) Sweet-liking is associated with transformation of heavy drinking into alcohol-related problems in young adults with high novelty seeking. Alcohol Clin Exp Res 38:2119–2126
Kampov-Polevoy AB, Garbutt JC, Janowsky D (1997) Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry 154:269–270
Kampov-Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E (2001) Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol 36:165–70
Karinen R, Andersen JM, Ripel Å, Hasvold I, Hopen AB, Mørland J, Christophersen AS (2009) Determination of heroin and its main metabolites in small sample volumes of whole blood and brain tissue by reversed-phase liquid chromatography-tandem mass spectrometry. J Anal Toxicol 33:345–350
Karlsson HK, Tuulari JJ, Tuominen L, Hirvonen J, Honka H, Parkkola R, Helin S, Salminen P, Nuutila P, Nummenmaa L (2016) Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Mol Psychiatry 21:1057–1062
Keskitalo K, Knaapila A, Kallela M, Palotie A, Wessman M, Sammalisto S, Peltonen L, Tuorila H, Perola M (2007) Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr 86:55–63
Lee MC, Wagner HN Jr, Tanada S, Frost JJ, Bice AN, Dannals DF (1988) Duration of occupancy of opiate receptors by naltrexone. J Nucl Med 29:1207–1211
Leknes S, Bastian B (2014) How does pain affect eating and food pleasure? Pain 155:652–653
Levine AS, Kotz CM, Gosnell BA (2003) Sugars and fats: the neurobiology of preference. J Nutr 133:831S–834S
Looy H, Callaghan S, Weingarten HP (1992) Hedonic response of sucrose likers and dislikers to other gustatory stimuli. Physiol Behav 52:219–225
Lugo RA, Kern SE (2002) Clinical pharmacokinetics of morphine. Journal of Pain and Palliative Care Pharmacotherapy 16:5–18
MacIntosh CG, Sheehan J, Davani N, Morley JE, Horowitz M, Chapman IM (2001) Effects of aging on the opioid modulation of feeding in humans. J Am Geriatr Soc 49:1518–1524
Mague SD, Blendy JA (2010) OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 108:172–182
Mahler SV, Berridge KC (2012) What and when to "want"? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology 221:407–426
McLeod RL, Parra LE, Mutter JC, Erickson CH, Carey GJ, Tulshian DB, Fawzi AB, Smith-Torhan A, Egan RW, Cuss FM (2001) Nociceptin inhibits cough in the Guinea-pig by activation of ORL1 receptors. Br J Pharmacol 132:1175–1178
Methven L, Xiao C, Cai M, Prescott J (2016) Rejection thresholds (RjT) of sweet likers and dislikers. Food Qual Prefer 52:74–80
Miotto K, McCann M, Basch J, Rawson R, Ling W (2002) Naltrexone and dysphoria: fact or myth? Am J Addict 11:151–160
Morley JE (1995) The role of peptides in appetite regulation across species. Am Zool 35:437–445
Morley JE, Parker S, Levine AS (1985) Effect of butorphanol tartrate on food and water consumption in humans. Am J Clin Nutr 42:1175–1178
Mysels DJ, Sullivan MA (2010) The relationship between opioid and sugar intake: review of evidence and clinical applications. Journal of Opioid Management 6:445–452
Nathan PJ, O'Neill BV, Bush MA, Koch A, Tao WX, Maltby K, Napolitano A, Brooke AC, Skeggs AL, Herman CS, Larkin AL, Ignar DM, Richards DB, Williams PM, Bullmore ET (2012) Opioid receptor modulation of hedonic taste preference and food intake: a single-dose safety, pharmacokinetic, and pharmacodynamic investigation with GSK1521498, a novel μ-opioid receptor inverse agonist. J Clin Pharmacol 52:464–474
Neale J, Nettleton S, Pickering L, Fischer J (2012) Eating patterns among heroin users: a qualitative study with implications for nutritional interventions. Addiction 107:635–641
Nolan LJ, Scagnelli LM (2007) Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst Use Misuse 42:1555–1566
Olsen MB, Jacobsen LM, Schistad EI, Pedersen LM, Rygh LJ, Roe C, Gjerstad J (2012a) Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor mu 1 gene: evidence of a sex and genotype interaction. J Neurosci 32:9831–9834
Olsen MB, Jacobsen LM, Schistad EI, Pedersen LM, Rygh LJ, Røe C, Gjerstad J (2012b) Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor mu 1 gene: evidence of a sex and genotype interaction. J Neurosci 32:9831–9834
Peciña M, Love T, Stohler CS, Goldman D, Zubieta J-K (2015) Effects of the mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology 40:957–965
Peciña S, Smith KS, Berridge KC (2006) Hedonic hot spots in the brain. Neuroscientist 12:500–511
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) nlme: Linear and Nonlinear Mixed Effects Model
Ray LA, Hutchison KE (2004) A polymorphism of the-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcoholism-Clinical and Experimental Research 28:1789–1795
Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M (1993) Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88:791–804
Scinska A, Koro E, Polanowska E, Kukwa A, Bogucka-Bonikowska A, Kostowski W, Habrat B, Bienkowski P (2000) An opioid receptor antagonist, naltrexone, does not alter taste and smell responses in humans. Pharmacol Rep 52:397–402
Smith YR, Zubieta J-K, Carmen MG, Dannals RF, Ravert HT, Zacur HA, Frost JJ (1998) Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. The Journal of Clinical Endocrinology & Metabolism 83:4498–4505
Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P (1995) A scale for the assessment of hedonic tone. The Snaith-Hamilton pleasure scale. Br J Psychiatry 167:99–103
Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD, Fields HL (2006) Endogenous opioids encode relative taste preference. Eur J Neurosci 24:1220–1226
Trenchard E, Silverstone T (1983) Naloxone reduces the food intake of normal human volunteers. Appetite 4:43–50
Verebey K, Volavka J, Mule SJ, Resnick RB (1976) Naltrexone: disposition, metabolism and effects after acute and chronic dosing. Clin Pharmacol Ther 20:315–328
Vindenes V, Handal M, Ripel Å, Boix F, Mørland J (2006) Conditioned place preference induced by morphine and morphine-6-glucuronide in mice. Pharmacol Biochem Behav 85:292–297
Weafer J, Burkhardt A, de Wit H (2014) Sweet taste liking is associated with impulsive behaviors in humans. Front Behav Neurosci 8:228
Wronski M, Skrok-Wolska D, Samochowiec J, Ziolkowski M, Swiecicki L, Bienkowski P, Korkosz A, Zatorski P, Kukwa W, Scinska A (2007) Perceived intensity and pleasantness of sucrose taste in male alcoholics. Alcohol Alcohol 42:75–79
Yeomans MR, Gray RW (1996) Selective effects of naltrexone on food pleasantness and intake. Physiol Behav 60:439–446
Yeomans MR, Gray RW (1997) Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol Behav 62:15–21
Yeomans MR, Gray RW (2002) Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev 26:713–728
Yeomans MR, Wright P (1991) Lower pleasantness of palatable foods in nalmefene-treated human volunteers. Appetite 16:249–259
Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M, Tao WX, Napolitano A, Skeggs AL, Brooke AC, Cheke L, Clayton NS, Sadaf Farooqi I, O'Rahilly S, Waterworth D, Song K, Hosking L, Richards DB, Fletcher PC, Bullmore ET (2013) Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry 18:1287–1293
Acknowledgments
We are grateful to Øivind Skare for help with setting up the mixed effects analyses. We thank Jeppe Riegels, Ingunn Olsen, Olga Chelnokova, and Hedda Gjertsen for help with data collection. We thank Elisabeth Øiestad, Vigdis Vindenes, and Liliana Bach at the Norwegian Institute for Public Health for help with determining medication doses and analyses of biological data. The study is indirectly supported by Aleris Healthcare, Norway, financing the research position of Frode Willoch at the University of Oslo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by grants from the Norwegian Research Council (ES455867) and a Ph.D. grant from the South-Eastern Norway Regional Health Authority (2013053).
Conflict of interests
The authors declare no competing or conflicting financial interests.
Electronic supplementary material
ESM 1
(RTF 5589 kb)
Rights and permissions
About this article
Cite this article
Eikemo, M., Løseth, G.E., Johnstone, T. et al. Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology 233, 3711–3723 (2016). https://doi.org/10.1007/s00213-016-4403-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4403-x