Abstract
Problems with ventilatory efficiency results in abnormal CO2 clearance. Measuring deadspace ventilation should be the most reliable method of monitoring ventilatory efficiency in mechanically ventilated patients. Since its first description by Bohr in the late 19th century to the current use of single-breath test for volumetric CO2, our understanding of the physiological deadspace has vastly improved. Yet indices of oxygenation seem to be the mainstay when instigating or fine-tuning ventilatory strategies. Deadspace and with it ventilatory efficiency has been largely forgotten. Recently though there has been a resurgence of interest in ventilatory efficiency. Several indices have been described that either predict deadspace or track ventilatory efficiency at the bedside. Thus making it more accessible and easy to monitor and study in large groups of patients, factors which have perhaps resulted in its under-utilisation in critical care. This review revisits the physiological concepts and methods of measuring deadspace. Described are the various clinical applications of deadspace measurements in the critically unwell. The potential reasons that have led to the variable being under-used are also examined. Finally we describe the indices currently available to track ventilatory efficiency at the bedside.
Similar content being viewed by others
Introduction
Alveolar ventilation is the amount of air per unit time that is involved in gas exchange. Ventilatory efficiency describes this volume as a proportion of the tidal volume. The remainder of the tidal volume is not involved in gas exchange and is conceptually known as the physiological deadspace. Whilst deadspace ventilation is probably of minimal consequence in normal conditions, its inherent clinical importance is that pathophysiological states of the lung result in its increase and hence a reduction in ventilatory efficiency. At the bedside this will manifest as altered carbon dioxide clearance. Such pathological processes are common in the critically ill and measurement of physiological deadspace should be a most valuable and intuitive tool to manage ventilation, yet it is seldom used.
The importance of carbon dioxide clearance was first appreciated in the Danish polio epidemic when clinicians rapidly realised that existing methods such as negative pressure ventilation resulted in deaths despite adequate oxygenation. The turning point was the realization that most deaths were due to inadequate ventilation [1, 2]. This led to the birth of positive pressure ventilation, respiratory physiological monitoring and with it intensive care medicine. The initial primacy of carbon dioxide clearance in monitoring during mechanical ventilation was key. Since then emphasis in practice has slowly changed and adequacy of oxygenation has replaced carbon dioxide as the principle focus for managing such patients. Yet the indirect role of ventilation in oxygenation is illustrated by the ability to oxygenate by insufflation. Carbon dioxide and its clearance has been considered of still lesser importance especially with the advent of permissive hypercapnia. This correctly identifies the relatively innocuous nature of mild hypercapnia but in doing so obscures the obvious fact that hypercapnia with adequate ventilation implies a significant increase in physiological deadspace with or without alterations in shunt. The pathophysiology underlying permissive hypercapnia is directly related to the underlying disease processes yet it is considered of secondary importance. It is a clinical paradox that while the concept of physiological deadspace has been known since the late 19th century, in the critical care setting it remains an underused and poorly understood variable.
It is time to revisit this neglected subject and the aims of this review are to examine current physiological concepts of deadspace, describe methods of measurement, and describe its common uses as a clinical tool in the critical care setting. Also examined are the current available methods to assess ventilatory efficiency at the bedside.
Terminology
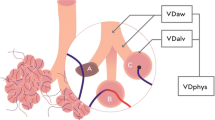
Deadspace is the portion of tidal volume that does not participate in gas exchange i.e. ‘wasted’. Physiological deadspace (VDphys) is a composed of airway deadspace (VDaw) and alveolar deadspace (VDalv):
In mechanically ventilated patients airway deadspace (VDaw) is the sum of anatomical deadspace (conducting airways) and apparatus deadspace. Alveolar deadspace is a construct that accounts for the remainder of the tidal volume that does not participate in gas exchange. In normal healthy subjects the alveolar deadspace is expected to be negligible.
The history of physiological deadspace
Bohr equation
Tidal volume (V T) is the sum of alveolar ventilation (V A) and physiological deadspace (V D):
In 1891 Bohr made two basic assumption when considering his approach to calculating deadspace [3]. Firstly a two-lung model was assumed whereby there was either gas exchange (alveolar ventilation) or deadspace. The ‘deadspace’ in this instance was assumed to be a fixed entity derived from previous cadaveric measurements (airway deadspace). Bohr proposed using Eq. 2 to calculate the alveolar volume. He also assumed that there was no carbon dioxide in inspired air. Therefore all carbon dioxide measured in mixed expired gas came from alveolar ventilation. The latter assumption is used in most current methods used to calculate deadspace.
Inserting the appropriate fractioned CO2 concentration (F) into each of the three volumes in Eq. 2 and given that the concentration of CO2 in deadspace gas \( ( {F_{{{\text{D}}_{{{\text{CO}}_{2} }} }} } ) \) is 0, using the conservation of mass principle Eq. 2 can be restated as:
From Eq. 2 we also know that V A = V T − V D therefore:
Solving for V D/V T we get:
where \( F_{{{\text{A}}_{{{\text{CO}}_{ 2} }} }} \) is the alveolar fractional concentration of CO2 and \( F_{{{\text{E}}_{{{\text{CO}}_{ 2} }} }} \)is the mixed expired fractional concentration of CO2. This equation was originally used by Bohr to obtain \( F_{{{\text{A}}_{{{\text{CO}}_{ 2} }} }} \) and the associated alveolar volume. Equation 5 is commonly known as Bohr’s equation.
Since then Eq. 5 is frequently restated substituting fractioned CO2 concentration with partial pressures of CO2 (PCO2):
where \( P_{{{\text{A}}_{{{\text{CO}}_{ 2} }} }} \) is the alveolar \( P_{{{\text{CO}}_{ 2} }} \) and \( P_{{{\text{E}}_{{{\text{CO}}_{ 2} }} }} \) is the mixed expired \( P_{{{\text{CO}}_{ 2} }} \). Subsequent to the work of Bohr it was recognized that deadspace was a varying entity. The introduction of gas analysis allowed the use of expired CO2 as a surrogate for alveolar CO2 [4]. Mixed expired CO2 was collected in a reservoir bag and thereby enabling the calculation of deadspace using Eq. 6 [5].
Enghoff modification of Bohr’s equation
One of the limitations of Bohr’s method of calculating deadspace has been accurately measuring alveolar PCO2 \( ( {P_{{{\text{A}}_{{{\text{CO}}_{ 2} }} }} } ) \). Given the inter-alveoli heterogeneity of ventilation-perfusion ratio and the uneven emptying of lung units due to varied time constants, end-tidal CO2 is an inaccurate representation of \( P_{{{\text{A}}_{{{\text{CO}}_{ 2} }} }} \) [6]. This is especially true in the case of diseased lungs where there is increased disparity in the homogeneity of the \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) matching and alveolar emptying. By assuming that there was no difference in the values of alveolar \( P_{{{\text{CO}}_{ 2} }} \) and arterial \( P_{{{\text{CO}}_{ 2} }} ( {P_{{{\text{a}}_{{{\text{CO}}_{ 2} }} }} } ) \), in 1938 Enghoff modified the Bohr deadspace equation by substituting \( P_{{{\text{A}}_{{{\text{CO}}_{ 2} }} }} \) with \( P_{{{\text{a}}_{{{\text{CO}}_{ 2} }} }} \) [7]. Accounting for this substitution we can restate Eq. 6:
Equation 7 is known as the Bohr–Enghoff equation and to this day remains the most commonly used method to calculate deadspace using mixed-expired CO2.
Fowler’s airway deadspace
Fowler’s novel method was to use continuous nitrogen gas analysis to calculate deadspace [8]. His subjects inhaled 99.6% oxygen and the expired volume and concentration of nitrogen were continuously measured. Fowler defined ‘physiological deadspace’ as the volume representing the conducting airways. He described it as the point where a large change in the gas composition occurred. Effectively this is the volume represented by what we now know as the airway deadspace. Fowler proposed analyzing the graph-form of the nitrogen volume-concentration of expired gas to calculate the deadspace (Fig. 1).
Since then Bartels and colleagues demonstrated that the deadspace for carbon dioxide was the same as that of nitrogen and Fowler’s methods could be applied to calculate deadspace using single breath analysis of expired CO2 [9].
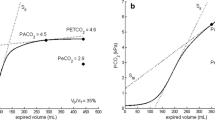
More recently Langley and colleagues plotted the expired CO2 volume against the expired total volume and described an alternative method of calculating airway deadspace [10]. This curvilinear graph is shown in Fig. 2. A straight best-fit line is extrapolated from the linear portion of the graph and the intercept of this line on the volume axis (x axis) is representative of the deadspace (Fig. 2). This method correlates with Fowler’s method for calculating VDaw but with the added advantage that it does not rely on the visual interpretation for determining equal areas [11, 12].
Langley’s method for calculating anatomical deadspace. Plotted is the volume of expired CO2 against the volume of expired gas. The dotted line represents the best-fit line of the linear segment of the graph. The point of interception of the dotted line on the x axis represents the airway deadspace volume
While several factors can influence airway deadspace, in the critical care setting this volume remains relatively unchanged. Large stepwise changes in airway deadspace in mechanically ventilated patients are usually as a result of equipment deadspace and hence easily quantifiable. Any changes in measured physiological deadspace, without added equipment deadspace, are mostly a result of changes in alveolar deadspace. It is alveolar deadspace and its inherent interaction with physiological deadspace that is clearly most important clinically.
Measuring physiological deadspace and ventilatory efficiency
Single breath test for carbon dioxide
The CO2 single breath test (SBT-CO2) of volumetric capnography can be used to extract useful information about ventilatory efficiency. Breath-by-breath analysis of expired CO2 concentrations plotted against the total expired volume allows for meaningful calculations of the area under the graph. The volumetric SBT-CO2 expirogram can be divided into three phases (Fig. 3). Phase I is the volume of gas that is in the airway (and the apparatus volume in ventilated patients). Phase II is composed of gas from the terminal airways and from the alveoli with the shortest transit times. Phase III is also known as the alveolar plateau and represents majority of the alveolar emptying.
The slopes of phase II and III can impart useful information on visual analysis. A flat alveolar plateau (phase III) would be indicative of homogenous lung emptying. Conversely an expirogram with an alveolar plateau with a steep continuous gradient represents heterogeneity in alveolar emptying. This is frequently encountered in diseased lung states. Inhomogeneity in alveolar emptying could either result from within the alveolar units where in the more distal alveoli there is likely to be lower \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) ratio or due to inter-unit heterogeneity [6].
Fletcher further analyzed the SBT-CO2 and gave a detailed proposal of measuring the various parts that contribute to physiological deadspace [13, 14]. Figure 4 shows that the area under the graph (x) is the volume of CO2 eliminated in a breath. The area ABCDA describes the maximum (hypothetical) volume of gas that could be excreted in the breath. ‘Efficiency’ which is the portion of volume participating in gas exchange is described as:
1 − efficiency represents the same fraction as deadspace. Therefore ‘E’ or efficiency can be restated as a product of more familiar terms:
where V A represents the alveolar volume (ml) and V E represents the expired tidal volume (ml).
In diseased lungs where there is significant \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) mismatch the peak \( P_{{{\text{CO}}_{ 2} }} \) of the alveolar plateau seldom equals alveolar \( P_{{{\text{CO}}_{ 2} }} \) or arterial \( P_{{{\text{CO}}_{ 2} }} \). Fletcher proposed using this discrepancy to calculate the alveolar deadspace. As shown in Fig. 5A a horizontal line is drawn from the point on the y axis that represents the arterial \( P_{{{\text{CO}}_{ 2} }} \). As per Fowler’s method a vertical line is drawn to elicit equal areas p and q. Area X once again represents the volume of CO2 eliminated, area Y represents the VDalv and area Z represents VDaw.
a Components of SBT-CO2 expirogram as described by Fletcher. Area X represents the CO2 elimination volume, area Y is the alveolar deadspace volume, and area Z is the airway deadspace volume, where areas p and q are equal. b Shows the SBT-CO2 expirogram for a mechanically ventilated patient undergoing elective surgery. c Shows the SBT-CO2 expirogram for a mechanically ventilated patient with COPD admitted to intensive care, the slope of phase III is steeper. d shows SBT-CO2 expirogram of a mechanically ventilated patient with ARDS, the transition between phase II and phase III is harder to define
Therefore from the analysis we can deduce that anatomical deadspace fraction is:
alveolar deadspace fraction is:
and physiological deadspace can be defined as:
SBT-CO2 deadspace calculations have been validated against the V bohrD in both animal models and humans [15, 16]. There are several advantages to using volumetric capnography (SBT-CO2) over the Douglas Bag method for measuring deadspace in mechanically ventilated patients. Collecting mixed expired gas in a Douglas bag is subject to inherent accuracies due to gas compression predominantly within the ventilator circuit [17, 18]. On expiration this compressed gas will dilute the mixed expired CO2 concentration in the bag, and therefore lower the measured PeCO2, resulting in falsely elevated physiological deadspace. Volumetric capnography measures the expired CO2 closer to the endotracheal tube and therefore the measurements do not require a correction factor accounting for the gas compression in the ventilator circuit. Additionally volumetric capnography provides breath to breath analysis of the CO2 capnograph and enables frequent assessment of deadspace changes.
Although recent developments in both software and capnography have made this method more accessible there continue to be technical difficulties in its use. These include adequate capturing of the waveform and differentiating between phase II and phase III of the curve especially when tidal volumes are small and gradient of phase III is steep [19]. Crucial to its utility is the definition of the transition point between phase II and phase III. Yet whether defined visually or calculated mathematically this point remains arbitrary and can be ambiguous [16]. Paradoxically as deadspace volume increases the reliability of the method is further compromised.
To overcome these shortcomings Romero et al. used the SBT-CO2 to propose an alternative method to analyse ventilatory efficiency. This moves away from the visual description of the transition from phase II to phase III. Instead, as in Fig. 2 they plot the expired volume of CO2 against the expired tidal volume to describe the term alveolar ejection volume (V AE) as the predicted point on the VCO2 curve where alveolar emptying begins. This volume attempts quantification of the phase III of the slope. The alveolar ejection volume versus tidal volume ratio (V AE/V t) [20] is an index that monitors alveolar heterogeneity and has been shown to correlate with severity of lung diseases as well as being a useful predictor of outcome [21, 22]. Limitations of this index as a clinical tool include the requirement of relatively simple software to calculate [22] the values and that it is not a previously described physiological term.
Current concepts in deadspace
Ventilation-perfusion mismatch
Physiological deadspace is a marriage of a tangible anatomical entity and an intangible physiological function that cannot be directly measured. Our understanding of deadspace and shunt has been largely influenced by the three lung model as proposed by Riley and Cournand [23, 24]. In this proposed model there are three compartments:
-
a)
The first compartment is both ventilated and perfused. This is the most efficient compartment.
-
b)
The second compartment lacks in ventilation and hence the blood supply to this compartment contributes to shunt.
-
c)
The final compartment is ventilated but is lacking in perfusion.
This model only defines three isolated points across a broad spectrum of ventilation and perfusion matching. In reality all factors that cause inequality of \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) mismatch will contribute to deadspace ventilation. Not all alveoli contributing to physiological deadspace are completely devoid of perfusion. Alveolar units with excess ventilation relative to perfusion will contribute to deadspace. Diseases such as chronic obstructive pulmonary disease or pulmonary fibrosis cause an increase in \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) mismatch due to defective alveolar gas mixing and inequalities in regional gas distribution [25–27]. Consequently there is heterogeneous \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) distribution within lung units and the entire lungs. Depending on the nature and severity of the pathology the levels of \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) mismatch can be vastly varied.
Shunt and physiological deadspace
The Bohr–Enghoff equation calculates physiological deadspace using the difference between the arterial and mixed-expired CO2, so any factor that influences arterial \( P_{{{\text{CO}}_{ 2} }} \) will result in altered physiological deadspace. Therefore physiological deadspace is influenced by shunt. If the \( P_{{{\text{CO}}_{ 2} }} \) of the venous admixture is large enough and the lung compensatory mechanisms are overwhelmed, the resultant elevation of arterial \( P_{{{\text{CO}}_{ 2} }} \) would increase the calculated physiological (Bohr–Enghoff) deadspace [14, 28]. Consequently physiological deadspace is not only influenced by a high \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) but also by a low \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \).
The influence of shunt on deadspace is non-linear. \( P_{{{\text{a}}_{{{\text{CO}}_{ 2} }} }} \) and consequently physiological deadspace is only affected in disease processes that result in large shunt. At shunt fractions (Q s/Q t) of 0.2 the influence of shunt on \( P_{{{\text{a}}_{{{\text{CO}}_{ 2} }} }} \) is negligible [29]. Due to the nature of the variables involved it is very difficult to directly measure the influence of shunt on physiological deadspace. Figure 6 shows the effects of shunt on \( P_{{{\text{a}}_{{{\text{CO}}_{ 2} }} }} \) and hence Bohr–Enghoff deadspace in a high-fidelity computer model [30]. Shunt fractions of greater than 0.5 shows a rapid increase in physiological deadspace [28, 31].
Relationship of pulmonary shunt and \( P_{{{\text{a}}_{{{\text{CO}}_{ 2} }} }} \) as proposed by a computerized model by Tang and colleagues [30]
Although shunt plays a role in increasing physiological deadspace, alveolar deadspace has a much more profound impact on CO2 elimination as illustrated by Fig. 7 [32]. Even though the contributions of shunt to alveolar deadspace are not strictly “deadspace”, it none-the-less represents an abnormality in gas exchange (CO2 elimination) and a pointer to abnormal pathology.
Comparison of the relative effects of shunt (a) and \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) (b) mismatch on calculated physiological deadspace. Log SDQ, second moment (dispersion) of the ventilation/perfusion distribution on a log scale. Figure recreated with permission from Wagner [32]
Pathophysiology of deadspace in ALI/ARDS
Pathological processes can alter measured physiological deadspace in many ways. For example in mechanically ventilated patients with severe ARDS, increased deadspace can result from increased alveolar deadspace as the tidal volume is distributed to poorly or non-perfused parts of the lungs. Over-distension of segments of the lung that are functioning normally either as a manifestation of using high peak end expiratory pressure (PEEP) or due to the use of high tidal volumes/ventilatory pressures would result in areas of relatively high \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) which would also increase the deadspace fraction. Another frequently encountered pathological features in ARDS is the presence of microemboli which can further result in \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) abnormalities. This is supported by the randomized control trial in which the use of activated protein C resulted in an associated improvement in physiological deadspace in patients with acute lung injury [33]. Elsewhere the ratio of angiopoietin 2 and 1, a proposed indicator of endothelial damage, has been shown to have a prognostic interaction with deadspace fraction in such patients. This suggests that endothelial damage and the resultant disruption in pulmonary microcirculation may also lead to increases in ventilation-perfusion mismatching in ALI [34]. Additionally, as the severity of illness increases the level of intra-pulmonary shunt increases, which would further contribute to the deadspace fraction. Theoretically a drop in cardiac output and a resultant drop in the pulmonary perfusion pressures could also contribute to raising the \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) ratio. In these situations positive pressure ventilation itself may actively redistribute ventilation according to local compliance and radically alter \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) ratio.
The current position of deadspace measurement in critical care practice
In the critical care setting carbon dioxide clearance or ventilatory efficiency is influenced by a limited number of factors which include ventilation, perfusion and to a lesser extent CO2 production. This contrasts with indices of oxygenation which are determined by numerous factors that are both intrinsic (pathological) and extrinsic (physics) to the body. These factors are frequently independent of ventilation. Studies on Mount Everest with impressively low oxygen indices in exercising climbers [35, 36] clearly demonstrate that the relationship of oxygenation to clinical well-being is relatively indirect. Nevertheless oxygenation continues to be used as the primary tool for the initiation and structuring of ventilatory strategies [37]. This may partly be due to the key but weak association between commonly used indices of oxygenation and survival [38]. Equally important is that it is not conventional practice to be guided by deadspace either whilst describing or monitoring the severity of pulmonary failure. It may be argued that whilst describing conditions such as such as ALI/ARDS it would be more relevant to also incorporate ventilatory efficiency for categorizing disease severity. As this is in effect unexplored, there is a reasonable reluctance amongst clinicians to use this most relevant of parameters.
Recently there has been a resurgence in interest in using deadspace measurements and in particular using SBT-CO2 in the clinical setting. Yet given the potential usefulness of ventilatory efficiency/deadspace the reasons for its absence deserve explanation:
-
1.
The methods to calculate deadspace as described above are either time-honoured but cumbersome (Douglas bag) or expensive and ancillary (volumetric capnography) [39]. There are only a few reliable automated commercially available methods of measuring deadspace and they are not yet fully integrated into standard monitoring systems (see Table 1).
Table 1 Some of the devices that are commercially available in Europe that measure dead space using volumetric capnography -
2.
As it is seldom measured there is a lack of clinical familiarity with the measurement and a therefore natural tendency to continue with a standard approach based on oxygenation.
-
3.
Similarly there is a paucity of information regarding interventions that directly manipulate deadspace and hence an assumption that it is of limited value.
-
4.
As it is rarely measured there are very few large studies describing its behaviour in critically unwell patients and its true value as a clinical tool has yet to be evaluated.
Indices of ventilatory efficiency
Recently there has been increasing interest in developing more simple ‘user-friendly’ indices to monitor changes in ventilatory efficiency at the bedside. These are summarized in Table 2. All the indices use variables measured at the bedside and broadly fall into 2 main categories. The first use complex calculations to predict numerical values for V d/V t. Unfortunately some of the assumed values inherent to either the method of derivation or the proposed calculations will inevitably lead to inaccuracies. The second do not offer explicit quantification of deadspace but rather look at tracking ventilatory efficiency using bedside variables. The latter whilst easier to reproduce and calculate would be subject to similar inaccuracies.
Most of these methods have yet to be adequately validated. The bedside method proposed by Saddiki et al. [40] uses the alveolar gas equation to estimate V d/V t. In order to obtain quantitative values of V d/V t they have used the Harris–Benedict equation to estimate values of VCO2 for a given patient. The Harris–Benedict equation is not only complex to calculate but also has been shown to be unreliable in the critical care setting [41, 42]. This brings into question the validity of the V d/V t values extracted from the equation.
An alternative approach from Frankenfield et al. uses regression analysis to derive a predictive equation to estimate V d/V t. The equation has been validated for patients in steady state with an FiO2 < 0.6. Although there is good correlation between measured and calculated values of V d/V t, this remains validated in a very small select group of patients. The difference between arterial and end-tidal (PaCO2-EtCO2) has been proposed to be used to either predict V alvd /V alvt using [43] or to track changes in V alvd /V alvt [44]. Once again neither methods have been validated in patients. Conceptually using EtCO2 as a tool to monitor deadspace is likely to lead to inaccuracies due to its dependence on the ventilatory patterns and tidal volumes.
Lastly ventilatory ratio (VR) uses similar principles as those applied in respiratory medicine to stipulate a predicted standard of ventilation to obtain an ideal PaCO2. Measured values are then compared to these values. Whilst this is a simple method that has been physiologically defined it remains to be validated as a clinical tool.
Clinical utility
In the critical care setting there are diverse but isolated examples of deadspace measurements being used as a clinical tool. In pulmonary embolism deadspace-tidal volume fraction in conjunction with D-Dimer assays, has been shown to be of diagnostic value [48–50]. Deadspace fraction has also been used to predict likelihood of successful extubation in paediatric patients [51]. It would seem likely that from first principles monitoring ventilatory efficiency could play a vital role in weaning and prediction of extubation. The negative impact of rising deadspace on weaning has been demonstrated in the neonatal population [52]. Although these population groups are distinct from adults, the pathophysiologcial principles that result in difficult weaning stand true in all populations. Currently the most widely used index to predict successful weaning is the frequency-tidal volume ratio and it focuses on the endurance of breathing [53]. This index is simple to calculate and shows high sensitivity but has low specificity. It does not incorporate the corresponding efficiency of CO2 elimination. A combination of the work of breathing and ventilatory efficiency would in theory provide a more refined index to predict successful weaning. Studies have demonstrated the superiority of such combined indices in small groups of patients resulting in increased specificity as compared to f/V T [54, 55]. Larger studies are needed to explore the advantages of using a combined index that intuitively appears to offer a more comprehensive evaluation of the pathophysiology of weaning failure.
Deadspace measurements have been used to assess the impact of varying PEEP on carbon dioxide elimination and to determine the optimum levels of PEEP during lung recruitment [56, 57]. The SBT-CO2 has been used to study the effects of PEEP on the 2 individual components of deadspace (VDaw and VDalv) in patients with acute lung injury [58]. This showed that there was no uniform response to PEEP.
In animal studies it has been shown that deadspace is highly specific and sensitive in monitoring lung collapse [59]. In these studies the lungs use a surfactant-depleted model and show large changes with recruitment techniques. This contrasts with the findings in adults with acute lung injury where recruitment is far less effective and excessive PEEP may exert a negative influence on \( {{\dot{V}} \mathord{\left/ {\vphantom {{\dot{V}} {\dot{Q}}}} \right. \kern-\nulldelimiterspace} {\dot{Q}}} \) matching secondary to overdistension [60]. Nevertheless lung recruitment manoeuvres have been evaluated using deadspace fraction or ventilatory efficiency [61–63]. It appears to be a more useful means of assessing the efficacy of recruitment than using indices of oxygenation [64].
Deadspace and acute lung injury
Perhaps of greatest interest and relevance in the critical care setting is the utility of deadspace measurements in the context of ALI, ARDS, and modes of ventilation. In patients with ARDS a range of pathophysiological mechanisms increase ventilatory inefficiency. Hence as one might anticipate deadspace measurements have been shown to be a useful prognostic marker for patients with ARDS. This has been clearly demonstrated in several studies where non-survivors have been shown to have a significantly higher deadspace ratio than survivors [40, 65–67]. Similarly using a measurement of ventilatory efficiency, V AE/V t, has been shown to be a useful predictor of outcome in patients with lung injury [22]. The timing and the magnitude of the deadspace abnormality has also been shown to predict the clinical outcome in patients with lung injury. While prognostic indicators have their place, measurements that allow both monitoring and manipulation of ventilatory management have more immediate clinical usefulness. Kallet and colleagues demonstrated that serial measurements of deadspace could be used to monitor disease progression in ARDS [68, 69]. Others have demonstrated its use in monitoring manoeuvres such as prone positioning that are designed to improve gas exchange [70, 71]. Patients with ALI/ARDS that demonstrate a decrease in arterial \( P_{{{\text{CO}}_{ 2} }} \) i.e. increased ventilatory efficiency on prone-positioning have shown improved survival compared to those patients that show no improvement in arterial \( P_{{{\text{CO}}_{ 2} }} \) [72]. Clearly measurement of ventilatory efficiency has a potential role in the management of ventilation.
There is more recently, linkage between prognosis and potential pathophysiological mechanisms underlying deadspace changes. Ong and colleagues have demonstrated a prognostic association between dead space fraction and the ratio of angiopoietin 2 and 1 which are proposed markers of endothelial damage. The interaction between deadspace and pulmonary capillary perfusion is clearly important and this may prove an exciting and fruitful new direction for clinical study [34].
In the rapidly evolving field of ventilatory management where modalities such as extracorporeal membrane CO2 removal and high frequency oscillatory ventilation are becoming increasing prevalent, a fresh look at our methods of monitoring gas exchange in ventilated patients is essential. CO2 clearance is a crucial part of these strategies and needs to be monitored. Routine monitoring of ventilatory efficiency could lead to more appropriate diagnostic categorization of respiratory failure in terms of CO2 clearance rather than just oxygenation. This may in turn be helpful in earlier instigation of these novel therapies. Additionally monitoring efficiency of CO2 clearance during treatment could aide in the management of the finer aspects of extracorporeal membrane CO2 removal such as assessing device efficacy and deciding the timeliness of return to more conventional ventilation.
The two common themes in these reports across a spectrum of clinical activity are that potential usefulness of measuring ventilatory efficiency has been demonstrated but to date investigation and application of these measurements are very limited. That should in itself be enough to stimulate interest across other areas of clinical practice.
Conclusion
Recently there has been a growing trend in the literature pointing to the use of deadspace measurement as a monitoring tool in mechanically ventilated patients. Whilst the introduction of more sophisticated and advanced measurement devices and software have increased our understanding of deadspace ventilation, it has not increased its use in intensive care medicine. Clearly if there were more affordable, simpler and integrated methods to track ventilatory efficiency our use of this measurement would increase and thereby enhance our understanding of it as a clinical tool. Early and repeated measurements in lung disease could provide clinicians with valuable information for prognostication and disease monitoring. Although several relatively easy to use bedside tools to monitor deadspace have been described in the literature they need to be validated in real-time against established methods of deadspace measurement but interest in these techniques is growing.
With the advent of extracorporeal gas exchange management of respiratory failure is rapidly changing and carbon dioxide clearance will return to the fore. Early detection of deadspace abnormalities may be useful for monitoring and optimizing ventilatory modes. This may lead to managing ventilation through assessment of ventilatory efficiency and potentially developing methods of influencing and manipulating deadspace. More importantly it may lead to new avenues in our understanding of the pathophysiology and progression of respiratory failure. Even if a fraction of these ideas come to fruition it would justify a resurgence in clinical interest in ventilatory efficiency. It is the authors’ contention that in the next decade critical care will rediscover the importance of carbon dioxide clearance.
References
Andersen EW, Ibsen B (1954) The anaesthetic management of patients with poliomyelitis and respiratory paralysis. Br Med J 1:786–788
Ibsen B (1954) The anaesthetist’s viewpoint on the treatment of respiratory complications in poliomyelitis during the epidemic in Copenhagen, 1952. Proc R Soc Med 47:72–74
Bohr C (1891) Ueber die Lungenathmung. Skand Arch Physiol 2:236–238
Haldane JS, Priestley JG (1905) The regulation of the lung-ventilation. J Physiol 32:225–266
Douglas CG, Haldane JS (1912) The capacity of the air passages under varying physiological conditions. J Physiol 45:235–238
Drummond GB, Fletcher R (2006) Deadspace: invasive or not? Br J Anaesth 96:4–7
Englhoff H (1938) Volumen inefecax. Bemerkungen zur Frage des schaedlichen Raumes. Uppsala La¨ k Fo¨ r Fo¨ rh 44:191–218
Fowler WS (1948) Lung function studies; the respiratory dead space. Am J Physiol 154:405–416
Bartels J, Severinghaus JW, Forster RE, Briscoe WA, Bates DV (1954) The respiratory dead space measured by single breath analysis of oxygen, carbon dioxide, nitrogen or helium. J Clin Invest 33:41–48
Langley FE, Duroux P, Nicolas RL, Cumming G (1975) Ventilatory consequences of unilateral pulmonary artery occlusion. Les Colloques de l’Institut National de la Santeet de la Recherche Medicale 51:209–212
Fletcher R (1984) Airway deadspace, end-tidal CO2, and Christian Bohr. Acta Anaesthesiol Scand 28:408–411
Blanch L, Romero PV, Lucangelo U (2006) Volumetric capnography in the mechanically ventilated patient. Minerva Anestesiol 72:577–585
Fletcher R, Jonson B (1984) Deadspace and the single breath test for carbon dioxide during anaesthesia and artificial ventilation. Effects of tidal volume and frequency of respiration. Br J Anaesth 56:109–119
Fletcher R, Jonson B, Cumming G, Brew J (1981) The concept of deadspace with special reference to the single breath test for carbon dioxide. Br J Anaesth 53:77–88
Riou Y, Leclerc F, Neve V, Dupuy L, Noizet O, Leteurtre S, Sadik A (2004) Reproducibility of the respiratory dead space measurements in mechanically ventilated children using the CO2SMO monitor. Intensive Care Med 30:1461–1467
Arnold JH, Thompson JE, Arnold LW (1996) Single breath CO2 analysis: description and validation of a method. Crit Care Med 24:96–102
Forbat AF, Her C (1980) Correction for gas compression in mechanical ventilators. Anesth Analg 59:488–493
Crossman PF, Bushnell LS, Hedley-Whyte J (1970) Dead space during artificial ventilation: gas compression and mechanical dead space. J Appl Physiol 28:94–97
Wenzel U, Wauer RR, Schmalisch G (1999) Comparison of different methods for dead space measurements in ventilated newborns using CO2-volume plot. Intensive Care Med 25:705–713
Romero PV, Lucangelo U, Lopez Aguilar J, Fernandez R, Blanch L (1997) Physiologically based indices of volumetric capnography in patients receiving mechanical ventilation. Eur Respir J 10:1309–1315
Blanch L, Lucangelo U, Lopez-Aguilar J, Fernandez R, Romero PV (1999) Volumetric capnography in patients with acute lung injury: effects of positive end-expiratory pressure. Eur Respir J 13:1048–1054
Lucangelo U, Bernabe F, Vatua S, Degrassi G, Villagra A, Fernandez R, Romero PV, Saura P, Borelli M, Blanch L (2008) Prognostic value of different dead space indices in mechanically ventilated patients with acute lung injury and ARDS. Chest 133:62–71
Riley RL, Cournand A (1949) Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol 1:825–847
Riley RL, Cournand A, Donald KW (1951) Analysis of factors affecting partial pressures of oxygen and carbon dioxide in gas and blood of lungs; methods. J Appl Physiol 4:102–120
Wagner PD, Naumann PF, Laravuso RB (1974) Simultaneous measurement of eight foreign gases in blood by gas chromatography. J Appl Physiol 36:600–605
Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB (1977) Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest 59:203–216
Yamaguchi K, Mori M, Kawai A, Takasugi T, Oyamada Y, Koda E (1997) Inhomogeneities of ventilation and the diffusing capacity to perfusion in various chronic lung diseases. Am J Respir Crit Care Med 156:86–93
Niklason L, Eckerstrom J, Jonson B (2008) The influence of venous admixture on alveolar dead space and carbon dioxide exchange in acute respiratory distress syndrome: computer modelling. Crit Care 12:R53
Kuwabara S, Duncalf D (1969) Effect of anatomic shunt on physiologic deadspace-to-tidal volume ratio—a new equation. Anesthesiology 31:575–577
Tang Y, Turner MJ, Baker AB (2005) Effects of alveolar dead-space, shunt and V/Q distribution on respiratory dead-space measurements. Br J Anaesth 95:538–548
Mecikalski MB, Cutillo AG, Renzetti AD Jr (1984) Effect of right-to-left shunting on alveolar dead space. Bull Eur Physiopathol Respir 20:513–519
Wagner PD (2008) Causes of a high physiological dead space in critically ill patients. Crit Care 12:148
Liu KD, Levitt J, Zhuo H, Kallet RH, Brady S, Steingrub J, Tidswell M, Siegel MD, Soto G, Peterson MW, Chesnutt MS, Phillips C, Weinacker A, Thompson BT, Eisner MD, Matthay MA (2008) Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med 178:618–623
Ong T, McClintock DE, Kallet RH, Ware LB, Matthay MA, Liu KD (2010) Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med 38:1845–1851
West JB, Hackett PH, Maret KH, Milledge JS, Peters RM Jr, Pizzo CJ, Winslow RM (1983) Pulmonary gas exchange on the summit of Mount Everest. J Appl Physiol 55:678–687
Grocott MP, Martin DS, Levett DZ, McMorrow R, Windsor J, Montgomery HE (2009) Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med 360:140–149
Gowda MS, Klocke RA (1997) Variability of indices of hypoxemia in adult respiratory distress syndrome. Crit Care Med 25:41–45
Ware LB (2005) Prognostic determinants of acute respiratory distress syndrome in adults: impact on clinical trial design. Crit Care Med 33:S217–S222
Badal JJ, Chen KJ, Loeb RG (2011) Measurement of Dead Space in Subjects Under General Anesthesia Using Standard Anesthesia Equipment. Anesth Analg 112(2):375–377
Siddiki H, Kojicic M, Li G, Yilmaz M, Thompson TB, Hubmayr RD, Gajic O (2010) Bedside quantification of dead-space fraction using routine clinical data in patients with acute lung injury: secondary analysis of two prospective trials. Crit Care 14:R141
Flancbaum L, Choban PS, Sambucco S, Verducci J, Burge JC (1999) Comparison of indirect calorimetry, the Fick method, and prediction equations in estimating the energy requirements of critically ill patients. Am J Clin Nutr 69:461–466
Frankenfield D, Hise M, Malone A, Russell M, Gradwell E, Compher C (2007) Prediction of resting metabolic rate in critically ill adult patients: results of a systematic review of the evidence. J Am Diet Assoc 107:1552–1561
Hardman JG, Aitkenhead AR (1999) Estimation of alveolar deadspace fraction using arterial and end-tidal CO2: a factor analysis using a physiological simulation. Anaesth Intensive Care 27:452–458
Hardman JG, Aitkenhead AR (2003) Estimating alveolar dead space from the arterial to end-tidal CO(2) gradient: a modeling analysis. Anesth Analg 97:1846–1851
Nunn JF, Hill DW (1960) Respiratory dead space and arterial to end-tidal carbon dioxide tension difference in anesthetized man. J Appl Physiol 15:383–389
Sinha P, Fauvel NJ, Singh S, Soni N (2009) Ventilatory ratio: a simple bedside measure of ventilation. Br J Anaesth 102:692–697
Frankenfield DC, Alam S, Bekteshi E, Vender RL (2010) Predicting dead space ventilation in critically ill patients using clinically available data. Crit Care Med 38:288–291
Kline JA, Israel EG, Michelson EA, O’Neil BJ, Plewa MC, Portelli DC (2001) Diagnostic accuracy of a bedside D-dimer assay and alveolar dead-space measurement for rapid exclusion of pulmonary embolism: a multicenter study. JAMA 285:761–768
Kline JA, Kubin AK, Patel MM, Easton EJ, Seupal RA (2000) Alveolar dead space as a predictor of severity of pulmonary embolism. Acad Emerg Med 7:611–617
Verschuren F, Liistro G, Coffeng R, Thys F, Roeseler J, Zech F, Reynaert M (2004) Volumetric capnography as a screening test for pulmonary embolism in the emergency department. Chest 125:841–850
Hubble CL, Gentile MA, Tripp DS, Craig DM, Meliones JN, Cheifetz IM (2000) Deadspace to tidal volume ratio predicts successful extubation in infants and children. Crit Care Med 28:2034–2040
Estay A, Claure N, D’Ugard C, Organero R, Bancalari E (2010) Effects of instrumental dead space reduction during weaning from synchronized ventilation in preterm infants. J Perinatol 30:479–483
Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324:1445–1450
Jabour ER, Rabil DM, Truwit JD, Rochester DF (1991) Evaluation of a new weaning index based on ventilatory endurance and the efficiency of gas exchange. Am Rev Respir Dis 144:531–537
Gluck EH (1996) Predicting eventual success or failure to wean in patients receiving long-term mechanical ventilation. Chest 110:1018–1024
Maisch S, Reissmann H, Fuellekrug B, Weismann D, Rutkowski T, Tusman G, Bohm SH (2008) Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anesth Analg 106:175–181 table of contents
Suter PM, Fairley B, Isenberg MD (1975) Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 292:284–289
Beydon L, Uttman L, Rawal R, Jonson B (2002) Effects of positive end-expiratory pressure on dead space and its partitions in acute lung injury. Intensive Care Med 28:1239–1245
Tusman G, Suarez-Sipmann F, Bohm SH, Pech T, Reissmann H, Meschino G, Scandurra A, Hedenstierna G (2006) Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med 32:1863–1871
Lucangelo U, Blanch L (2004) Dead space. Intensive Care Med 30:576–579
Tusman G, Bohm SH, Sipmann FS, Maisch S (2004) Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg 98:1604–1609 table of contents
Tusman G, Bohm SH, Suarez-Sipmann F, Turchetto E (2004) Alveolar recruitment improves ventilatory efficiency of the lungs during anesthesia. Can J Anaesth 51:723–727
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Calzia E, Radermacher P, Bein T (2006) Unveiling alveolar recruitment: the fascinating trail between theory and practice. Intensive Care Med 32:1686–1688
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Shimada Y, Yoshiya I, Tanaka K, Sone S, Sakurai M (1979) Evaluation of the progress and prognosis of adult respiratory distress syndrome. Simple respiratory physiologic measurement. Chest 76:180–186
Raurich JM, Vilar M, Colomar A, Ibanez J, Ayestaran I, Perez-Barcena J, Llompart-Pou JA (2010) Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care 55:282–287
Valta P, Uusaro A, Nunes S, Ruokonen E, Takala J (1999) Acute respiratory distress syndrome: frequency, clinical course, and costs of care. Crit Care Med 27:2367–2374
Kallet RH, Alonso JA, Pittet JF, Matthay MA (2004) Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care 49:1008–1014
Johannigman JA, Davis K Jr, Miller SL, Campbell RS, Luchette FA, Frame SB, Branson RD (2000) Prone positioning for acute respiratory distress syndrome in the surgical intensive care unit: who, when, and how long? Surgery 128:708–716
Davis K Jr, Johannigman JA, Campbell RS, Marraccini A, Luchette FA, Frame SB, Branson RD (2001) The acute effects of body position strategies and respiratory therapy in paralyzed patients with acute lung injury. Crit Care 5:81–87
Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, Valenza F, Caironi P, Pesenti A (2003) Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 31:2727–2733
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, P., Flower, O. & Soni, N. Deadspace ventilation: a waste of breath!. Intensive Care Med 37, 735–746 (2011). https://doi.org/10.1007/s00134-011-2194-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2194-4