Abstract
Purpose
Passive leg raising (PLR) is a maneuver performed to test the cardiac Frank-Starling mechanism. We assessed the influence of PLR-induced changes in preload on the performance of PLR-induced change in pulse pressure (ΔPLRPP) and cardiac output (ΔPLRCO) for fluid responsiveness prediction.
Methods
Sedated, nonarrhythmic patients with persistent shock were included in this prospective multicenter study. Cardiac output and pulse pressure were measured at baseline (patient supine), during PLR (lower limbs lifted to 45°) and after 500-ml volume expansion. Patients were classified as responders or not.
Results
In the whole population (n = 102), the area under the receiver-operating characteristic curve (AUC) was 0.76 for ΔPLRPP and was higher for ΔPLRCO (0.89)(p < 0.05), but likelihood ratios were close to 1. In patients with a PLR-induced increase in central venous pressure (CVP) of at least 2 mmHg (n = 49), ΔPLRPP and ΔPLRCO disclosed higher AUCs than in the rest of the population (0.91 vs. 0.66 and 0.98 vs. 0.83; p < 0.05); positive/negative likelihood ratios were 9.3/0.14 (8% cutoff level) and 30/0.07 (7% cutoff level), respectively.
Conclusions
A PLR-induced change in CVP ≥2 mmHg was required to allow clinical usefulness of PLR-derived indices. In this situation, ΔPLRPP performed well for predicting fluid responsiveness in deeply sedated patients.
Similar content being viewed by others
Introduction
In case of persistent circulatory failure after initial resuscitation, intensivists often use additional volume expansion with the aim of increasing cardiac output (CO). However, nearly half of the patients in shock are not responsive to volume expansion [1–3] after the first hours of care, and an unnecessary volume load may be deleterious [4]. After the initial phase of resuscitation, clinical examination, cardiac volumes and filling pressures [central venous pressure (CVP); pulmonary artery occlusion pressure (PAOP)] are of little interest to predict the response to volume expansion [5]. Therefore, new indices have been developed using passive leg raising (PLR) or heart-lung interactions [6].
Because of its simplicity and its safety due to the theoretical reversibility, PLR is an appealing test for predicting fluid responsiveness [7]. It mimics volume expansion by shifting blood from the capacitance veins of the legs towards the intrathoracic compartment [8], leading to an increase in right [9] and left [7] ventricular preload and then in left ventricular stroke volume (SV) and in CO, if the patient’s ventricles work on the ascending portion of their Frank-Starling curve [2, 7]. As CO measurement may be cumbersome, PLR-induced changes in various SV surrogates have been evaluated for the prediction of volume responsiveness, with PLR-induced changes in pulse pressure (PP) being the easiest SV surrogate to record at the bedside (ΔPLRPP), but poor performance has been reported [2, 10]. We hypothesized that ΔPLRPP performance may be downplayed mainly by the failure of the PLR maneuver to significantly increase cardiac preload. As a change in CVP reflects that the Frank-Starling mechanism of the heart has been tested [6, 11–14], ΔPLRPP could be a reliable index among patients increasing their CVP during PLR.
The aim of this study was to assess the influence of PLR-induced changes in CVP on the performance of ΔPLRPP and ΔPLRCO for the prediction of fluid responsiveness.
Patients and methods
Participating centers were the Hôpital Bichat-Claude Bernard of Paris, the Centre Hospitalier Régional Universitaire of Tours and the Centre Hospitalier Régional of Orléans, France.
Patients
Adult patients undergoing volume-controlled mechanical ventilation without triggering the ventilator were included if they exhibited acute circulatory failure (systolic BP <90 mmHg, mean BP <65 mmHg, skin mottling, urine output <0.5 ml/kg/h, arterial lactate >2.5 mmol/l or vasopressor infusion), a Ramsay score >4 and no arrhythmia.
Patients were not included in case of diuretic treatment, uncontrolled hemorrhage, brain death, leg amputation, phlebitis, compression stockings, intra-aortic balloon pump support, severe hypoxemia (PaO2/FiO2 <70 mmHg), high risk of pulmonary edema [body weight indexed extravascular lung water (EVLWi) >22 ml/kg (PiCCO™ system: Pulsion Medical Systems AG™, Munich, Germany)] or transmural PAOP >18 mmHg (PAOPtm, estimated via the measurement of respiratory changes in PAOP [15]; pulmonary artery catheter: Edwards Lifesciences™, Irvine, CA).
The study procedure was stopped in case of changes in respirator settings or vasoactive therapy, occurrence of arrhythmia or respiratory intolerance to volume expansion [EVLWi >22 ml/kg or PAOPtm >22 mmHg or 5% decrease in pulse oxymetry (SpO2)].
Mechanical ventilation, vasoactive therapy, sedation and paralysis were set by the attending physician and not modified.
Our regional ethics review board approved the study protocol. Patients were included either after written informed consent from a relative or after emergency enrollment followed by delayed consent [16].
Study protocol
The study was divided into five phases: baseline 1; 45° PLR (the lower limbs were lifted in a straight manner to 45°, with the trunk and the bed being kept horizontal [7]); return to baseline position (baseline 2); 300 ml over 18 min (to assess the respiratory tolerance) and an additional 200-ml volume expansion (modified fluid gelatin) over 12 min (Fig. 1) [7].
Measurements
One minute after the beginning of each study phase, we started to measure invasive BP, heart rate (HR), SpO2, CVP, PAOP (end-expiratory value, measured on frozen waveform), total positive end-expiratory pressure (PEEPt) and CO.
CO was measured through end-expiratory injection of 10 ml or 15 ml (transcardiac and transpulmonary thermodilution, respectively) of an iced dextrose solution [using a closed injection system with in-line temperature measurement: CO-set+™ system (Edwards Lifesciences, Irvine, CA) or included in the PiCCO™ system]. Three consecutive measurements within 10% (if not, seven measurements) were averaged.
The correct placement of the pulmonary artery catheter was ascertained by visualization of concordant waveforms and calculation of the respiratory changes in PAOP-to-respiratory changes in the PAP ratio [17].
CVP, PAOP and BP were measured with a disposable transducer (TruWave™, Baxter™ Division Edwards, Maurepas, France), zeroed at the level of the mid-axillary line. Four recordings of BP, 60-s apart, were performed by “at a glance” direct readings of the value displayed by the monitor, regardless of the respiratory cycle. We calculated change in PP (average of four measurements) and CO induced by PLR (ΔPLRPP and ΔPLRCO):
Using off-line, high-resolution paper tracings, including airway and BP waveforms, and after their numerical enlargement, “expiratory ΔPLRPP” was also calculated, based on end-expiratory measurements of PP, in order to avoid any influence of respiratory changes in BP. The absence of any spontaneous respiratory motion and of any triggering of the respirator was verified in each patient by (1) clinical observation, (2) similar settled and measured respiratory rates, and (3) no negative accident on airway pressure recording.
An echocardiography was performed within 6 h of measurements in order to quantify valvular regurgitations or intracardiac shunts.
Statistical analysis
After volume expansion, patients were classified as responders if CO increased by more than 10% and as nonresponders otherwise. This cutoff value was chosen because a measured 9% change in CO (which we rounded to 10%) reliably reflects that a real change has taken place [18]. To validate this choice in our patients (assessment of inter-measurement variability within each set of measurements), we calculated the least significant change for each set of CO measurements in each patient at each phase [(1.96√2)Cv/√number of measurements within one set] with Cv = coefficient of variation (SD/mean). This allowed us to ascertain that each individual patient classified as a responder had a CO increase above his/her individual least significant change [19]. We also made all the calculations using a 15% cutoff value to define fluid responsiveness [2].
For each index, we calculated the area under the receiver-operating characteristic curve (AUC), and determined the best cutoff value (Youden method) [20] and its corresponding positive and negative likelihood ratio (LR+ and LR−) [21]. As proposed, the values of 5 and 10 for the LR+ (or 0.2 and 0.1 for the LR−) helped to divide the continuous scale of LR+ and LR− into three categories: weak, good and strong evidence of discriminative power [21]. AUC comparisons were made between the different indices [22] and between different sub-groups [23]. The objective was to compute the AUC, LR+ and LR− of ΔPLRPP and ΔPLRCO in the whole population and in the predefined subgroups of patients with a CVP increase ≥2 and <2 mmHg during PLR [6]. The cutoff value of 2 mmHg was chosen because a change of this magnitude (1) can be identified with confidence on most monitors and recording devices, and (2) ascertains that the Frank-Starling mechanism has been tested [6, 11, 12]. Variables [expressed as mean ± SD or n (%)] were compared using Student's t test and Fisher's exact test (between responders and nonresponders), paired Student's t test (within each patient), analysis of variance and Scheffé test (between subgroups of patients) and analysis of variance or the chi-square test (between centers).
A value of p < 0.05 was considered significant. All statistical tests were two-tailed, performed using MedCalc® (Mariakerke, Belgium) and Statview® (SAS Institute, Cary, NC).
Results
One hundred two patients were included (Table 1).
The mean least significant change of CO measurements was 6.4 and 6.1% at baseline and after volume expansion, respectively.
Forty-three (42%) patients were responders after 500 ml volume expansion. They all exhibited individual CO changes from baseline to volume expansion greater than their individual least significant change.
Volume expansion was interrupted in two patients after 300 ml intolerance; data after 300 ml volume expansion were used for analysis.
In the whole population, HR and MAP were similar between baselines 1 and 2.
Hemodynamic parameters at baseline and their evolution after volume expansion are detailed in Table 2.
No difference between centers was noted concerning the proportion of responders, the Simplified Acute Physiology Score II, baseline MAP, HR, CO, ΔPLRPP and ΔPLRCO (all p > 0.05).
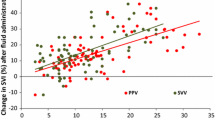
In the whole population, the AUC of ΔPLRCO for predicting fluid responsiveness was significantly higher than the AUC of ΔPLRPP [0.89 (0.81–0.94) and 0.76 (0.67–0.84), respectively, p = 0.007]. The best cutoff values were 7 and 9% for ΔPLRCO and ΔPLRPP, respectively (LR+ of 7.8 and 3.3 and LR− of 0.23 and 0.43, respectively) (Fig. 2).
Individual values of static and PLR-derived indices in responders and nonresponders of the whole study population. PLR passive leg-raising, CVP central venous pressure at baseline, PAOP pulmonary artery occlusion pressure at baseline, Δ PLR PP PLR-induced change in pulse pressure, Δ PLR CO PLR-induced change in cardiac output (thermodilution measurement). Responders are defined as patients increasing their cardiac output by at least 10% after 500-ml volume expansion
Influence of CVP increase during PLR
Among the 48 patients (16 responders) with a minimal CVP increase of 2 mmHg during PLR, ΔPLRPP exhibited an AUC of 0.91 (0.79–0.97), with LR+ and LR− of 9.3 and 0.14, respectively (8% cutoff value) (Fig. 3). Otherwise, ΔPLRPP disclosed a lower AUC [0.66 (0.51–0.78), p = 0.002] (Fig. 4). Hemodynamic and respiratory parameters according to CVP changes during PLR are detailed in Table 3 and ESM1.
Performance of ΔPLRPP according to PLR-induced preload increase. Legend: Receiver-operating characteristic (ROC) curve obtained for passive leg raising (PLR)-induced increase in pulse pressure (ΔPLRPP) to predict a 10% increase in cardiac output after 500-ml volume expansion. PLR-induced preload increase denotes a central venous pressure increase of at least 2 mmHg during PLR compared to baseline 1. The area under the curve (AUC) in case of a marked preload increase with PLR was greater than when PLR did not sufficiently increase central venous pressure (p = 0.002)
ΔPLRCO also performed better in patients with a CVP increase ≥2 mmHg: AUC of 0.98 (0.89–1), and LR+ and LR− of 30 and 0.07, respectively (7% cutoff value) (Fig. 3). Otherwise, ΔPLRCO disclosed a lower AUC [0.83 (0.71–0.92), p = 0.018].
Similar results were found (see ESM2):
-
1.
when excluding (a) patients carrying either a pulmonary artery catheter with significant (grade III or IV) tricuspid regurgitation (n = 2) or a PiCCO™ system with a significant mitral and/or tricuspid and/or aortic regurgitation (n = 1) and (b) patients who did not receive 500 ml fluid loading (n = 2) (data not shown);
-
2.
when considering only the first PP measurement instead of averaging four measurements to calculate ΔPLRPP;
-
3.
when considering end-expiratory PP measurement (as measured on paper tracing) to calculate ΔPLRPP;
-
4.
when considering PLR-induced changes in SV instead of ΔPLRCO;
-
5.
when using a 15% cutoff value (instead of 10%) for fluid loading-induced increase in CO to define fluid responsiveness.
Static indices
CVP, PAOP and PAOPtm disclosed an AUC of 0.61 (0.50–0.71), 0.70 (0.56–0.83) and 0.69 (0.54–0.82), respectively, with all LRs being within the weak evidence range of values (LR+ below 5, LR− above 0.2) (Fig. 2).
Discussion
The main finding of this multicenter study of 102 patients in shock is that it highlights a physiological limitation of PLR-derived indices, i.e., the lack of PLR-induced change in preload. Thus, provided that CVP increases by at least 2 mmHg during PLR, ΔPLRCO and, remarkably, ΔPLRPP appear to be useful tools to predict fluid responsiveness in our population.
Our study showed that ΔPLRCO was the best index in our population. This strongly supports the validity of the physiological rationale of PLR to predict volume responsiveness.
Most other studies addressing PLR did not measure CO using the thermodilution technique and therefore did not evaluate this index, but showed results compatible with ours: ΔPLRPP is, overall, of low performance for detecting fluid responsiveness, whereas other left ventricular SV surrogates (aortic blood flow as assessed by esophageal Doppler, iliac artery pulse contour-derived CO) perform better [2, 10, 24–26].
Different mechanisms may explain the poor performance of PP as a SV surrogate during PLR, none of which can be firmly incriminated or excluded based on the data of our study or of others: (1) PLR may induce sympathetic or cardiac reflex stimulation, but this is unlikely in our deeply sedated patients as we did not observe significant HR changes during PLR [27, 28]. (2) Using PP as a surrogate of SV assumes a constant arterial tree compliance, which may not always be the case during PLR (see ESM3 for details). In our study, as the performance of ΔPLRPP has been shown to be good in a subgroup of patients, one may consider that PLR-induced changes in compliance were insufficient to downplay the performance of ΔPLRPP significantly. (3) The transferred blood from the legs towards the central compartment by our PLR technique may be insufficient in some patients to alter cardiac preload (e.g., in case of profound hypovolemia). Of note, this confounding factor can be controlled in the clinical setting by ascertaining that the Frank-Starling mechanism of the heart has been tested by PLR. Indeed, in patients fulfilling a change of CVP of at least 2 mmHg during PLR, the performance of PLR-derived indices was significantly higher than in other patients. This validity criterion was underlined in recent international guidelines [6], but surprisingly has never been reported in order to improve the performance of PLR-derived indices. In this favorable circumstance, ΔPLRPP >8% or <8% provides good evidence in favor of (LR+ = 9.3) or against (LR− = 0.14) fluid responsiveness, respectively.
Maximizing blood transfer during PLR may be another way to improve ΔPLRPP performance. Another technique for PLR has been proposed, consisting of a postural change from a semi-recumbent to a supine position [2, 10]; this causes a higher increase in cardiac preload [29]. However, the performance of ΔPLRPP was similar in our whole population compared to studies using this semi-recumbent-to-supine PLR technique [2, 10]. Both PLR techniques need to be compared within one population of responders and nonresponders to draw definite conclusions about their relative performances.
We considered that changes in CVP reflect changes in preload and, as recommended [6], that a PLR-induced change in CVP of at least 2 mmHg denotes that the Frank-Starling mechanism has been tested [11–13, 30]. Actually, right ventricle preload assessment is controversial and complex [31], and a basic measurement of CVP is clearly not a good indicator of cardiac preload [31]. However, transmural CVP, i.e., the CVP relative to the pressure surrounding the heart (the pleural pressure), is an important determinant of cardiac preload [13]. We did not measure transmural CVP, but one can reliably hypothesize that PLR-induced changes in CVP (measured with a device that is zeroed relative to atmospheric pressure) reflect changes in transmural CVP (and then in right ventricle preload), provided that changes in pleural pressure did not occur. Interestingly, PLR-induced changes in the plateau pressure and PEEPt were negligible in our population (mean change of 0.6 ± 0.5 and 0.2 ± 0.2 cmH2O, respectively), suggesting that PLR-induced changes in pleural pressure were clinically insignificant. Further, the significant difference we observed for the performance of PLR-derived indices according to the PLR-induced changes in CVP strongly supports our hypothesis.
ΔPLRCO was the best performing index in our study as it allowed a very strong prediction of fluid responsiveness (LR+ = 30) and a strong prediction against it (LR− = 0.07) in case of an increase of CVP ≥2 mmHg during PLR. Nevertheless, CO measurement during PLR is cumbersome to carry out (bolus injections), and even if simpler means of CO determination exist, none is readily available at the bedside like ΔPLRPP. Indeed, the latter only requires an arterial and a central venous line, and is simply performed by direct reading of the displayed value of CVP and PP at baseline and after 1 min of PLR. Of note, one of the main results of our study is that ΔPLRPP is useful to predict fluid responsiveness provided that one ascertains that the PLR maneuver has altered the patient preload (increase in CVP ≥2 mmHg). The use of a “blind” PLR maneuver, i.e., with no concurrent CVP measurement, may explain the poor performance reported until now for ΔPLRPP [2, 10].
Of note, the best cutoff value for ΔPLRCO (7%, derived from ROC curve analysis) is lower than the cutoff value we used to discriminate between responders and nonresponders (10%). However, it was higher than our mean least significant change for thermodilution CO measurements (close to 6%), and when choosing a cutoff value of 10% for ΔPLRCO, we found similar results (LR+ and LR− of 12 and 0.39, respectively) in the whole population.
Table 3 exhibits a quite unexpected result: MAP significantly increased after volume infusion in nonresponders. Actually, it is likely that some patients may not have been stable enough during the study protocol and that spontaneous changes in MAP may have occurred, independently of fluid (un)responsiveness, as neither CO nor PP changed. In addition, due to the threshold chosen for the definition of fluid responsiveness, some patients classified as nonresponders may have in fact had a true increase (but below the threshold) in SV after volume expansion, which could explain a slight increase in PP and MAP.
As previously shown [5], neither CVP nor PAOP was a reliable predictor of fluid responsiveness in our study. Further, as in the only study addressing PAOPtm for volume responsiveness prediction [1], we showed that PAOPtm did not perform better than PAOP, suggesting the role of other mechanisms than extramural pressure to explain this poor predictive performance (nonlinear ventricular compliance, influence of the systolic function on the Frank-Starling curve).
Our patients were not studied in the very early phase of shock. Indeed, baseline CVP (11 ± 4 mmHg) and PAOP (12 ± 4 mmHg) were in the non-discriminatory range of values [6], showing that our patients had already undergone initial resuscitation. It is precisely for these patients, with persistent circulatory failure despite initial resuscitation and exhibiting noninformative values of static indices, that reliable dynamic indices are required, such as ΔPLRCO, ΔPLRPP or other indices based on respiratory variations of left ventricular SV [6]. Of note, most of the limitations noted for the latter (e.g., magnitude of tidal volume [1] and heart-to-respiratory rate ratio [32]) may not impact ΔPLRCO and ΔPLRPP. To eliminate any influence of heart-lung interaction on ΔPLRPP, we evaluated the performance of “expiratory ΔPLRPP,” i.e., based on end-expiratory measurements of PP. Interestingly, in our population, this rigorous approach of ΔPLRPP did not improve the performance of ΔPLRPP only based on either the mean of four collected measurements of PP “at a glance” or even a single one, suggesting that the influence of heart-lung interactions and thus ventilatory settings on ΔPLRPP performance is rather low, and probably negligible.
Importantly, as fluid challenge was very well tolerated in the short term during our study, and even if patients with very high risk of pulmonary edema were not included, performing carefully monitored fluid challenges [33] seems a valuable option when PLR-derived indices are not applicable (PLR-induced increase in CVP <2 mmHg), which was the case in half of our patients. Moreover, our population was deeply sedated, in sinus rhythm, under controlled ventilation. Whether our findings apply to other populations has to be investigated.
Conclusion
In our population, the variability of PLR-induced change in cardiac preload limited the usefulness of PLR-derived indices, but if one ascertained that PLR had increased CVP by at least 2 mmHg, ΔPLRPP (with a cutoff value of 8%) was a useful index to predict fluid responsiveness.
References
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL (2005) Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 31:517–523
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 34:1402–1407
Michard F, Teboul JL (2000) Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care 4:282–289
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL (2007) Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 35:64–68
Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, Meduri GU, Moreno RP, Putensen C, Stewart T, Torres A (2007) Hemodynamic monitoring in shock and implications for management. International consensus conference, Paris, France, 27–28 April 2006. Intensive Care Med 33:575–590
Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G (2002) Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest 121:1245–1252
Rutlen DL, Wackers FJ, Zaret BL (1981) Radionuclide assessment of peripheral intravascular capacity: a technique to measure intravascular volume changes in the capacitance circulation in man. Circulation 64:146–152
Thomas M, Shillingford J (1965) The circulatory response to a standard postural change in ischaemic heart disease. Br Heart J 27:17–27
Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL (2009) Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med 37:951–956
Magder S (2005) The use of central venous pressure in critically ill patients. In: Pinsky MR, Payen D (eds) Functional hemodynamic monitoring. Springer, Berlin, pp 299–311
Magder S (2005) How to use central venous pressure measurements. Curr Opin Crit Care 11:264–270
Magder S (2006) Central venous pressure: a useful but not so simple measurement. Crit Care Med 34:2224–2227
Magder S (2006) Central venous pressure monitoring. Curr Opin Crit Care 12:219–227
Teboul JL, Pinsky MR, Mercat A, Anguel N, Bernardin G, Achard JM, Boulain T, Richard C (2000) Estimating cardiac filling pressure in mechanically ventilated patients with hyperinflation. Crit Care Med 28:3631–3636
Law 2004-806 (August 11, 2004). Official Journal of the French Republic. Article 89
Teboul JL, Besbes M, Andrivet P, Axler O, Douguet D, Zelter M, Lemaire F, Brun-Buisson C (1992) A bedside index assessing the reliability of pulmonary occlusion pressure measurements during mechanical ventilation with positive end-expiratory pressure. J Crit Care 7:22–29
Ostergaard M, Nilsson LB, Nilsson JC, Rasmussen JP, Berthelsen PG (2005) Precision of bolus thermodilution cardiac output measurements in patients with atrial fibrillation. Acta Anaesthesiol Scand 49:366–372
Lodder MC, Lems WF, Ader HJ, Marthinsen AE, van Coeverden SC, Lips P, Netelenbos JC, Dijkmans BA, Roos JC (2004) Reproducibility of bone mineral density measurement in daily practice. Ann Rheum Dis 63:285–289
Akobeng AK (2007) Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 96:644–647
Grimes DA, Schulz KF (2005) Refining clinical diagnosis with likelihood ratios. Lancet 365:1500–1505
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Lafanechere A, Pene F, Goulenok C, Delahaye A, Mallet V, Choukroun G, Chiche JD, Mira JP, Cariou A (2006) Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Crit Care 10:R132
Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M (2007) Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med 33:1133–1138
Thiel SW, Kollef MH, Isakow W (2009) Non-invasive stroke volume measurement and passive leg raising predict volume responsiveness in medical ICU patients: an observational cohort study. Crit Care 13:R111
London GM, Pannier BM, Laurent S, Lacolley P, Safar ME (1990) Brachial artery diameter changes associated with cardiopulmonary baroreflex activation in humans. Am J Physiol 258:H773–H777
Caille V, Jabot J, Belliard G, Charron C, Jardin F, Vieillard-Baron A (2008) Hemodynamic effects of passive leg raising: an echocardiographic study in patients with shock. Intensive Care Med 34:1239–1245
Jabot J, Teboul JL, Richard C, Monnet X (2009) Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 35:85–90
Magder S (1998) More respect for the CVP. Intensive Care Med 24:651–653
Squara P, Journois D, Estagnasie P, Wysocki M, Brusset A, Dreyfuss D, Teboul JL (1997) Elastic energy as an index of right ventricular filling. Chest 111:351–358
De Backer D, Taccone FS, Holsten R, Ibrahimi F, Vincent JL (2009) Influence of respiratory rate on stroke volume variation in mechanically ventilated patients. Anesthesiology 110:1092–1097
Vincent JL, Weil MH (2006) Fluid challenge revisited. Crit Care Med 34:1333–1337
Acknowledgments
Regional grant: Projet hospitalier de recherche clinique no. R10-5, Direction Régionale de la Recherche Clinique Région Centre, Tours, France.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented in part at the annual meeting of the Société de Réanimation de Langue Française, Paris, January 2007 (Réanimation 2007; 16:S046) and at the annual meeting of the American Thoracic Society, San Francisco, May 2007 (Am J Respir Crit Care Med 2007; 175:A33).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakhal, K., Ehrmann, S., Runge, I. et al. Central venous pressure measurements improve the accuracy of leg raising-induced change in pulse pressure to predict fluid responsiveness. Intensive Care Med 36, 940–948 (2010). https://doi.org/10.1007/s00134-010-1755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1755-2