Abstract
Purpose
Extracorporeal CO2 removal is an effective procedure to allow a protective ventilatory strategy in ARDS patients, but it is technically challenging due to the high blood flow required. Increasing the CO2 transfer through the membrane lung (ML) may lower the demand of extracorporeal blood flow and consequently allow for a wider clinical application of this technique. Since only the dissolved CO2 (5% of the total CO2 content) is easily removed by the ML, we tested whether acidifying the blood entering the ML to convert bicarbonate ions towards dissolved CO2 could enhance the CO2 transfer though the ML.
Methods
Six pigs were connected to an extracorporeal circuit comprising a ML. The extracorporeal blood flow was 500 ml/min, while the gas flow was 10 l/min. A 15-min continuous infusion of 0.5 N lactic acid was added to the extracorporeal blood flow before the ML at a rate of 1, 2 and 5 mEq/min. Between steps we waited for a reequilibration time of at least 30 min.
Results
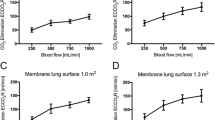
Acid infusion at 0, 1, 2 and 5 mEq/min increased pCO2 (56.19 ± 7.92, 68.24 ± 11.73, 84.28 ± 11.17 and 136.66 ± 18.46 mmHg, respectively) and decreased pH (7.39 ± 0.05, 7.30 ± 0.05, 7.20 ± 0.05 and 6.91 ± 0.05, respectively). ML CO2 removal increased 11, 23 and 70% during acid infusion at 1, 2 and 5 mEq/min, respectively.
Conclusions
Blood acidification at the inlet of a ML with infusion of 1, 2 and 5 mEq/min of lactic acid can increase the CO2 removal capacity of the ML up to 70%.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is a severe clinical syndrome characterized by hypoxaemia and decreased respiratory system compliance [1]. Mechanical ventilation may further damage the lung of ARDS patients leading to ventilator-induced lung injury (VILI) [2]. Protective ventilatory strategies aimed at reducing VILI are based on tidal volumes lower than 6 ml/kg and plateau airway pressures lower than 28 cm H2O. An effective protective ventilation, even tolerating hypercapnia, may be difficult to achieve in the more severe patients, since alveolar over distension may occur despite the use of low tidal volumes [3]. In such patients a substantial fraction (say, higher than 50%) of the body CO2 production may be extracorporeally removed through a membrane lung (ML) to allow an effective protective ventilation [4, 5]. To achieve this goal, however, the available technology requires an extracorporeal blood flow of at least 1 l/min [6, 7]. This is due to the fact that just the physically dissolved CO2 can cross the artificial lung membranes, and this is less than 10% of the total blood CO2 content. Such a large extracorporeal blood flow, which requires a large bore cannulas, is technically challenging and may be associated with side effects limiting the clinical application of extracorporeal CO2 removal (ECCO2R). Increasing the CO2 transfer through the membrane lung (ML) may lower the demand of extracorporeal blood flow and therefore decrease the complications related to this technique.
Since the majority of blood CO2 is chemically combined with water to form bicarbonate ion (see Eq. 1), we tested whether by acidifying the blood entering the ML, and therefore shifting the equilibrium from bicarbonate ion towards dissolved CO2 (and consequently increasing pCO2 and the transmembrane gradient), we could enhance the CO2 transfer capacity of the ML. In doing so, we expected to be able to remove the CO2 production of a human adult with a blood flow as low as 500 ml/min.
We present a proof of this concept in a preliminary experimental study in swine. We studied the effect upon the ML CO2 removal capacity of scalar acidification rates. We aimed at identifying the relationship between acid load and CO2 removal through the membrane lung.
Materials and methods
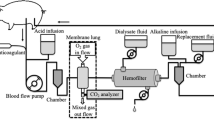
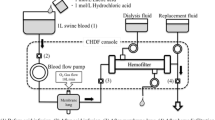
We sedated, tracheotomized and mechanically ventilated six female pigs (weight 37 ± 2 kg). We maintained anesthesia with propofol. A femoral artery and the external right jugular vein were cannulated for pressure monitoring, blood gas analysis and drug infusion. A Foley catheter was introduced into the bladder to measure urinary flow and internal body temperature. Before surgical preparation, 500 mg of ciprofloxacin was administered. The left femoral and jugular veins were surgically cannulated, respectively, with 12- and 15-F cannula for connection to a veno-venous extracorporeal circuit. The extracorporeal circuit consisted of a Jostra rotaflow centrifugal pump and a Quadrox D ML (Maquet, Hechingen, Germany). Immediately before the centrifugal pump chamber a small catheter, to be used for the acid infusion, was introduced into the circuit through a side port; the tip of the catheter was positioned at the entrance of the pump chamber to allow an almost instantaneous mixing between blood and the acid. The extracorporeal blood flow was set to 500 ml/min while the gas side was ventilated with 10 l/min of oxygen. The extracorporeal circuit was thermostated at 38°C. An activated clotting time value 50% above the baseline was maintained by continuous infusion of heparin. After surgical preparation, a 200-ml airway dead space was added, and the ventilator was set to obtain a mild hypercapnia (TV 430 ± 42 ml, RR 10 ± 4, FiO2 40%, PEEP 10, PaCO2 47 ± 5 mmHg). ECG, systemic arterial pressure, central venous pressure, urinary flow and bladder temperature were continuously monitored.
Lactic acid (0.5 N) was infused at rates of 1, 2 and 5 mEq/min in successive runs of 15 min each, separated by an interval of at least 30 min. The entire three-run procedure was then repeated after 1 h interruption. Each of the two runs in all studied animals was considered as a different experiment, for a total of 12 experiments. The CO2 removed by the ML (ml/min) was calculated as the partial pressure of CO2 of the gases exiting the ML (mmHg, measured by a capnograph, Datex-Ohmeda 4700) divided by 713 (mmHg), and multiplied by the gas flow (ml/min). Before starting each acid infusion (baseline) and after 10 min of acid infusion, we measured the CO2 removed by the ML, and we obtained blood samples from the artery and the extracorporeal circuit, before and after the injection point of the acid and after the ML for blood gas analysis. Plasma-free hemoglobin, as a marker of hemolysis, was measured before starting the extracorporeal circulation (baseline value) and at the end of each acid infusion.
Statistics
Data are expressed as mean ±SD. A level of p < 0.05 was considered significant. One-way analysis of variance for repeated measurements with Bonferroni correction for post hoc tests was used to evaluate the effect of different acid infusion (SPSS 15, Chicago, IL).
Results
Physiological parameters and arterial blood gas values at baseline were not statistically different throughout the study (see Table 1).
pH, pCO2, HCO3 −, BE and lactate at baseline and during acid infusion of blood withdrawn before and after the injection point of the acid and after the ML are shown in Table 2. Baseline values were not statistically different throughout the study (p > 0.13). As expected, incremental loads of l-lactic acid led to incremental reductions in pH and increases in pCO2, while transiting through the ML resulted in reductions of pCO2 and consequently increases in pH.
The CO2 removed by the ML increased with increasing acid loads from a baseline of 104 ± 20 to 121 ± 21, 131 ± 24 and 171 ± 32 ml/min, during 1, 2 and 5 mEq/min l-lactic acid infusion, respectively (see Fig. 1).
Plasma levels of free hemoglobin did not change during each acid infusion (baseline: 177 ± 58 μg/ml; 171 ± 32, 160 ± 37 and 143 ± 19 μg/ml, respectively, during 1, 2 and 5 mEq/min l-lactic acid infusion).
Discussion
This study demonstrates that blood acidification at the inlet of a ML, converting the blood bicarbonate into physically dissolved CO2, can significantly increase the CO2 removal by the ML. Several investigators have been testing various approaches to enhance CO2 removal from a low extracorporeal blood flow. In 1987, Snider et al. [8] infused 2–8 mEq/min of l-lactic acid (0.25 and 0.5 N) at the inlet of a ML in an awake sheep connected to a venous arterial by-pass (blood flow 500 ml/min and gas flow 4 l/min) and showed an increase of 120–170% in CO2 transfer rate from a baseline of 60 ml/min. However, visible hemolysis was present during infusion of 8 mEq/min of 0.5 N lactic acid. By using a lower acidification rate (5 vs. 8 mEq/min of lactic acid), we obtained comparable increases of CO2 transfer. The newer ML Quadrox D, a polymethylpentene hollow-fiber ML, performed better in terms of absolute CO2 transfer with a baseline value of 104 ml/min: 70% higher than the SCI-MED II SM-35 used by Snider. Furthermore, we observed no hemolysis during the whole experiment. Livigni et al. [9] presented a low-flow ECCO2R system comprising a hemofilter and a ML. This system allowed a 20% reduction of PaCO2 in a sheep model. No data on absolute CO2 transfer or blood pH were however reported.
In our study, the maximum CO2 removal was achieved at the highest acid infusion rate (5 mEq/min). The resulting pH drop was constantly around 0.5 pH units, bringing the pH to 6.9, which is reasonably safe since a similar pH level can be measured in human muscle capillaries during heavy exercise [10]. As expected, the decrease in pH caused an increase in pCO2, which reached levels as high as 140 mmHg. Despite these high levels, the outlet pCO2 was always lower than 25 mmHg, primarily due to an extremely efficient ML combined with a transit time of 30 s.
On the other hand, the high efficiency of the ML and the long transit time caused the pH at the outlet of the ML, during baseline non-acidified conditions, to reach a level as high as 7.85 ± 0.09, which may be very dangerous for blood cells. Adding an acid not only increased the CO2 removal, but also buffered the respiratory alkalosis resulting in a safer level of pH at the ML outlet. This is a proof of concept, acute study aimed at identifying a safe but effective range of acid infusion sufficient to obtain substantial CO2 removal from a low extracorporeal blood flow. Lactic acid was chosen as a physiological metabolite and substrate for many tissues. Knowing the target acid load required to achieve the desired CO2 removal, further laboratory investigations will be required to devise a strategy applicable for long-term application; particularly, it will be mandatory to study the systemic effects of sustained acid infusion. Possible strategies might use different acids and/or additional techniques to remove the acid load directly from the extracorporeal circuit.
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury—lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM (2007) Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 175:160–166
(2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342(18):1301–1308
Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, Iapichino G, Romagnoli G, Uziel L, Agostoni A et al (1986) Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA 256:881–886
Conrad SA, Zwischenberger JB, Grier LR, Alpard SK, Bidani A (2001) Total extracorporeal arteriovenous carbon dioxide removal in acute respiratory failure: a phase I clinical study. Intensive Care Med 27:1340–1351
Kolobow T, Gattinoni L, Tomlinson T, White D, Pierce J, Iapichino G (1977) The carbon dioxide membrane lung (CDML): a new concept. Trans Am Soc Artif Intern Organs 23:17–21
Snider MT, Chaudhari SN, Richard RB, Whitcomb DR, Russell GB (1987) Augmentation of CO2 transfer in membrane lungs by the infusion of a metabolizable organic acid. ASAIO Trans 33:345–351
Livigni S, Maio M, Ferretti E, Longobardo A, Potenza R, Rivalta L, Selvaggi P, Vergano M, Bertolini G (2006) Efficacy and safety of a low-flow veno-venous carbon dioxide removal device: results of an experimental study in adult sheep. Crit Care 10:R151
Geers C, Gros G (2000) Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80:681–715
Acknowledgments
This work was supported by the Italian Ministry of Universities and Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
The procedure described here is part of a blood processing technique for which patents are pending, International Patent Application: PCT/EP 2008/003661, 7 May 2008 and Italy: MI2007A000913, 7 May 2007.
Rights and permissions
About this article
Cite this article
Zanella, A., Patroniti, N., Isgrò, S. et al. Blood acidification enhances carbon dioxide removal of membrane lung: an experimental study. Intensive Care Med 35, 1484–1487 (2009). https://doi.org/10.1007/s00134-009-1513-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1513-5