Abstract
Case presentation
Despite chemoprophylaxis with isoniazid a 58-year-old Creole patient with mild rheumatoid arthritis developed disseminated tuberculosis, pulmonary aspergillosis and cutaneous herpes simplex infection during treatment with infliximab and methotrexate.
Treatment
The patient received antituberculous drugs (ethambutol, isoniazid, pyrazinamide, rifampicin), amphotericin B, flucytosine, and valaciclovir, along with prolonged intensive care treatment and mechanical ventilation.
Conclusions
The present case confirms that isoniazid prophylaxis (300 mg once daily, during 6 months) does not protect against the reactivation and dissemination of latent tuberculosis. It also shows that combined treatment with infliximab and methotrexate may induce severe immunosuppression with prolonged leukocytopenia and depressed cellular immunity, leading to multiple opportunistic infections. Extensive diagnostic testing, early start of antimicrobial therapy and enteral immunonutrition, and further infection prevention with selective decontamination of the digestive tract may have been the key to a good clinical outcome.
Similar content being viewed by others
Introduction
Active tuberculosis may develop soon after the initiation of treatment with infliximab, a humanized murine monoclonal antibody against tumor necrosis factor (TNF) α. Of the patients with infliximab-associated tuberculosis 56% have extrapulmonary symptoms, and 24% have disseminated disease [1]. Accumulating evidence suggests that TNF-α also plays a crucial role in the response to fungal pathogens, including Aspergillus fumigatus [2]. Since the worldwide approval of infliximab for rheumatoid arthritis and Crohn’s disease an increasing number of fungal infections have been reported, including disseminated coccidioidomycosis, cryptococcosis, histoplasmosis, Pneumocystis carinii pneumonia, pulmonary aspergillosis, and systemic Candida infections [1, 3]. Recently infliximab has been associated with the reactivation of viral infections such as varicella zoster virus, molluscum contagiosum, and cytomegalovirus [4, 5, 6]. We present the case of a patient who developed three opportunistic infections during therapy with infliximab and methotrexate, requiring prolonged mechanical ventilation and intensive care treatment.
Case report
A 58-year-old Creole woman was admitted to the intensive care unit of another hospital because of somnolence, severe hyponatremia (101 mmol/l), fever, bilateral pulmonary infiltrates, and respiratory failure. Her medical history included mild rheumatoid arthritis for which she received infliximab (10 mg/kg, every 2 months) and methotrexate (7.5 mg/week) in a clinical trial for 14 months. Since she had a positive (13 mm) tuberculin skin test before the start of therapy without signs or symptoms of active tuberculosis, she received isoniazid (INH) for 6 months (300 mg daily). Initial Gram staining and culture of the sputum yielded growth of Haemophilus influenzae, Escherichia coli, and Citrobacter freundii. After initial improvement she developed respiratory failure and needed ventilatory support and was transferred to our unit.
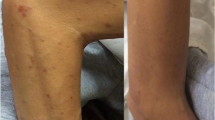
On admission she was comatose with a right-sided hemiparesis; there was disseminated intravascular coagulation, pancytopenia, and shock. Because an additional opportunistic pulmonary infection was suspected, bronchoalveolar lavage (BAL) was performed. Ziehl-Neelsen staining was positive (+5). Thrombocytopenia and disseminated intravascular coagulation prohibited lumbal puncture. Treatment consisted of intravenous volume expansion, inotropic support, mechanical ventilation, selective decontamination of the digestive tract, cefotaxim, ciproxin, enteral immunonutrition (Impact), antituberculous drugs (ethambutol, INH, pyrazinamide and rifampicin), and corticosteroids [7, 8]. Since the patient had persistent high fever and developed progressive pulmonary infiltrates and multiple ulcerations on the lower abdomen and genitals, a second BAL was performed on day 17, which showed growth of A. fumigatus. Herpes simplex virus type 2 was cultured from the abdominal and genital ulcerations but not from the BAL specimens. Skin biopsy findings supported the diagnosis of genital herpes. Further treatment consisted of amphotericin B, flucytosine, and later valaciclovir.
On admission, leukocytopenia was noted (3.1×109/l, 78% neutrophil granulocytes) with the following deficiencies: CD4+ count: 0.25×109/l (0.56–1.55); CD4+%: 49 (35–74); CD8+ count: 0.15×109/l (0.31–1.0); CD8+%: 30 (17–52); CD4+/CD8+ ratio: 1.63 (0.81–3.0). Analysis included bone marrow aspiration and culture. Test for human immunodeficiency virus antibodies was negative. Ziehl-Neelsen staining of the sputum remained positive for 5 weeks. The Mycobacterium tuberculosis, which was cultured from BAL fluid and bone marrow, was sensitive to the administered antituberculous drugs. The concentrations of amphotericin B, flucytosine, and antituberculous drugs were within the therapeutic range. The neurological state improved very slowly. In addition to the hemiparesis, there was severe critical illness polyneuropathy. She was discharged after 63 days of intensive care treatment and 54 days of mechanical ventilation. Her score on the Acute Physiology and Chronic Health Evaluation II was 31 (predicted mortality 0.73).
Several weeks later she was transferred to a rehabilitation center. However, after 1 week she was readmitted because of dyspnea and arthritis. After 4 months of treatment with antituberculous drugs M. tuberculosis was cultured from a metacarpal-phalangeal joint of the right hand.
Discussion
A variety of cytokine-based strategies are being explored for the treatment of chronic inflammatory diseases such as Crohn’s disease and rheumatoid arthritis and more recently for sepsis [9]. Given the complexity of cytokine interactions and the multiplicity of cytokine targets the effectiveness and toxicity of cytokine-based interventions are difficult to predict. Disseminated infections may arise, especially the reactivation of latent tuberculosis and herpes viruses, and the development of fungal infections. This risk is greatly increased in patients with concurrent immunosuppression, such as corticosteroids, azathioprine, methotrexate, and mercaptopurine [1, 3].
The present case is important for several reasons. First, the finding of a community acquired bacterial pneumonia with common pathogens does not exclude the coexistence of tuberculosis. Secondly, the treatment of latent tuberculosis (formerly called “chemoprophylaxis”) with a 6-month INH regimen does not always protect against the reactivation and dissemination of tuberculosis [10]. It has been demonstrated that among patients who take more than 80% of the prescribed doses the 6-month regimen decreases the incidence of active tuberculosis by 69% and the 12-month regimen by 93% compared with the 3-month regimen [11]. Therefore INH alone is not the gold standard for prophylaxis in high-risk patients. In retrospect, our patient had taken more than 80% of the prescribed dose, according to data from the referring hospital.
Thirdly, combination therapy with infliximab and methotrexate may cause a severe form of immunosuppression with multiple opportunistic infections due to prolonged leukocytopenia and depressed cellular immunity. The global reduction in CD4+ and CD8+ T-lymphocytes that we observed in our patient has been reported before [5, 12]. On the other hand, prolonged critical illness itself, especially disseminated tuberculosis, may cause pancytopenia and CD4+ T-lymphocytopenia, thereby creating additional immunosuppression which may further contribute to the development of other (fungal) infections [13, 14]. Since granulocytopenia was not observed (defined as a neutrophil count of <500×109/l), treatment with granulocyte or granulocyte-macrophage colony-stimulating factor was not instituted.
Finally, since there is currently no specific immune-enhancing therapy available, treatment of these patients should consist of general supportive measures, organ replacement therapy, appropriate anti-microbial therapy, early enteral immunonutrition, and infection prevention with selective decontamination of the digestive tract to avoid Gram-negative ventilator associated pneumonia [15]. After withdrawal of infliximab the immunodepressant effects persist for many months [1]. Therefore antituberculous therapy should probably be continued as long as the immunodepressant effects last [16]. In the absence of prospective data resumption of infliximab therapy is not recommended. Both physicians and patients should be aware of these side effects.
Conclusion
Combination therapy with infliximab and methotrexate may cause various opportunistic infections. In addition to supportive measures and specific antimicrobial therapy early enteral immunonutrition and other infection preventive measures may be of crucial importance in the treatment of these patients. Therapy with four first-line antituberculous drugs (INH, rifampicin, pyrazinamide, and ethambutol) may be needed for more than 8 weeks because of severe and prolonged immunosuppression.
References
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM (2001) Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med 345:1098–1104
Schelenz S, Smith DA, Bancroft GJ (1999) Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med Mycol 37:183–194
Warris A, Bjørneklett A, Gaustad P (2001) Invasive pulmonary aspergillosis associated with infliximab therapy. N Engl J Med 344:1099–1100
Baumgart DC, Dignass AU (2002) Shingles following infliximab infusion. Ann Rheum Dis 61:661
Cursiefen C, Grunke M, Dechant C, Antoni C, Junemann A, Holbach LM (2002) Multiple bilateral eyelid molluscum contagiosum lesions associated with TNF-alpha-antibody and methotrexate therapy. Am J Ophthalmol 134:270–271
Helbling D, Breitbach TH, Krause M (2002) Disseminated cytomegalovirus infection in Crohn’s disease following anti-tumor necrosis factor therapy. Eur J Gastroenterol Hepatol 14:1393–1395
De Gans J, Van de Beek D (2002) Dexamethasone in adults with bacterial meningitis. N Engl J Med 347:1549–1556
Prasad K, Volmink J, Menon GR (2000) Steroids for treating tuberculous meningitis. Cochrane Database Syst Rev 3:CD002244
Reinhart K, Menges T, Gardlund B, Zwaveling JH, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S, Tschaikowsky K, Brase R, Damas P, Kupper H, Kempeni J, Eiselstein J, Kaul M (2001) Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med 29:765–769
Hernandez-Cruz B, Ponce-de-Leon-Rosales S, Sifuentes-Osornio J, Ponce-de-Leon-Garduno A, Diaz-Jouanen E (1999) Tuberculosis prophylaxis in patients with steroid treatment and systemic rheumatic diseases. A case-control study. Clin Exp Rheumatol 17:81–87
Small PM, Fujiwara PI (2001) Management of tuberculosis in the United States. N Engl J Med 345:189–200
Baert FJ, D’Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, Rutgeerts PJ (1999) Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn’s ileocolitis. Gastroenterology 116:22–28
Kony SJ, Hane AA, Larouze B, Samb A, Cissoko S, Sow PS, Sane M, Maynart M, Diouf G, Murray JF (2000) Tuberculosis-associated severe CD4+ T-lymphocytopenia in HIV-seronegative patients from Dakar. SIDAK Research Group. J Infect 41:167–171
Zaharatos GJ, Behr MA, Libman MD (2001) Profound T-lymphocytopenia and cryptococcemia in a human immunodeficiency virus-seronegative patient with disseminated tuberculosis. Clin Infect Dis 33:E125–E128
Sanchez Garcia M, Cambronero Galache JA, Lopez Diaz J, Cerda Cerda E, Rubio Blasco J, Gomez Aguinaga MA, Nunez Reiz A, Rogero Marin S, Onoro Canaveral JJ, Sacristan del Castillo JA (1998) Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled, multicenter trial. Am J Respir Crit Care Med 158:908–916
Salmon D (2002) Recommendations about the prevention and management of tuberculosis in patients taking infliximab. Joint Bone Spine 69:170–172
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Klooster, J.M., Bosman, R.J., Oudemans-van Straaten, H.M. et al. Disseminated tuberculosis, pulmonary aspergillosis and cutaneous herpes simplex infection in a patient with infliximab and methotrexate. Intensive Care Med 29, 2327–2329 (2003). https://doi.org/10.1007/s00134-003-1867-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1867-z