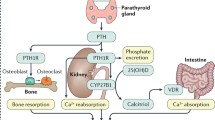
Abstract
Hypoparathyroidism is a disorder characterized by hypocalcemia, deficient PTH, and abnormal bone remodeling. Standard treatment of hypoparathyroidism consists of oral calcium and vitamin D supplementation. However, maintaining serum calcium levels can be a challenge. In addition, concerns exist regarding hypercalciuria and ectopic calcifications that can be associated with such treatment. Hypoparathyroidism is the only classic endocrine deficiency disease for which the missing hormone, PTH, is not yet an approved treatment. This review focuses on the use of PTH in the treatment of hypoparathyroidism, in the form of teriparatide [PTH(1–34)] and the full-length molecule, PTH(1–84). Studies in hypoparathyroid subjects demonstrate that PTH(1–34) and PTH(1–84) lower or abolish supplemental calcium and vitamin D requirements as well as increase markers of bone turnover. Densitometric and histomorphometric studies in some subjects treated with PTH(1–34) and PTH(1–84) show an improvement in bone-remodeling dynamics and return of bone metabolism toward normal levels. Given the chronic nature of hypoparathyroidism, and the expectation that PTH will be used for extended periods of time in hypoparathyroidism, further studies are needed to determine the long-term safety of PTH therapy in this population.
Similar content being viewed by others
References
Bilezikian JP, Khan A, Potts JT Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 2011, 26: 2317–37.
Cusano NE, Rubin MR, Sliney J Jr, Bilezikian JP. Mini-review: new therapeutic options in hypoparathyroidism. Endocrine 2012, 41: 410–4.
Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008, 359: 391–403.
Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012, 97: 4507–14.
Pearce SH, Williamson C, Kifor O, et al. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calciumsensing receptor. N Engl J Med 1996, 335: 1115–22.
Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S. A novel mutation of the signal peptide of the preproparathyroid hormone gene associated with autosomal recessive familial isolated hypoparathyroidism. J Clin Endocrinol Metab 1999, 84: 3792–6.
Arlt W, Fremerey C, Callies F, et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol 2002, 146: 215–22.
Cusano NE, Rubin MR, McMahon DJ, et al. The effect of PTH(1–84) on quality of life in hypoparathyroidism. J Clin Endocrinol Metab 2013, 98: 2356–61.
Sikjaer T, Rolighed L, Ortenblad N, et al. PTH (1–84) Substitution Therapy in Hypoparathyroidism: Effects on Muscle Cells, Muscle Function, Postural Stability and Quality of Life. In: Program of the 34th Annual Meeting of the American Society of Bone and Mineral Research. Minneapolis, MN SA0141: 2012.
Rubin MR, Dempster DW, Zhou H, et al. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res 2008, 23: 2018–24.
Abugassa S, Nordenstrom J, Eriksson S, Sjoden G. Bone mineral density in patients with chronic hypoparathyroidism. J Clin Endocrinol Metab 1993, 76: 1617–21.
Touliatos JS, Sebes JI, Hinton A, et al. Hypoparathyroidism counteracts risk factors for osteoporosis. Am J Med Sci 1995, 310: 56–60.
Rubin MR, Dempster DW, Kohler T, et al. Three dimensional cancellous bone structure in hypoparathyroidism. Bone 2010, 46: 190–5.
Rubin MR, Dempster DW, Sliney J Jr, et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res 2011, 26: 2727–36.
Sikjaer T, Rejnmark L, Rolighed L, et al. The effect of adding PTH(1–84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res 2011, 26: 2358–70.
Albright F, Ellsworth R. Studies on the physiology of the parathyroid glands: I. Calcium and Phosphorus Studies on a Case of Idiopathic Hypoparathyroidism. J Clin Invest 1929, 7: 183–201.
Mazziotti G, Bilezikian J, Canalis E, et al. New understanding and treatments for osteoporosis. Endocrine 2012, 41: 58–69.
Winer KK, Yanovski JA, Cutler GB Jr. Synthetic human parathyroid hormone 1–34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA 1996, 276: 631–6.
Winer KK, Yanovski JA, Sarani B, Cutler GB Jr. A randomized, crossover trial of once-daily versus twice-daily parathyroid hormone 1–34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab 1998, 83: 3480–6.
Winer KK, Ko CW, Reynolds JC, et al. Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab 2003, 88: 4214–20.
Winer KK, Sinaii N, Peterson D, et al. Effects of once versus twice-daily parathyroid hormone 1–34 therapy in children with hypoparathyroidism. J Clin Endocrinol Metab 2008, 93: 3389–95.
Winer KK, Sinaii N, Reynolds J et al. Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1–34 versus calcitriol and calcium. J Clin Endocrinol Metab 2010, 95: 2680–8.
Winer KK, Zhang B, Shrader JA, et al. Synthetic human parathyroid hormone 1–34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 2011, 97: 391–9.
Gafni RI, Brahim JS, Andreopoulou P, et al. Daily parathyroid hormone 1–34 replacement therapy for hypoparathyroidism induces marked changes in bone turnover and structure. J Bone Miner Res 2012, 27: 1811–20.
Rubin MR, Sliney J Jr, McMahon DJ, et al. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int 2010, 21: 1927–34.
Sikjaer T, Rejnmark L, Thomsen JS, et al. Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH(1–84): a randomized controlled study. J Bone Miner Res 2012, 27: 781–8.
Shoback D, Clarke B, Brandi ML, et al. Safety and tolerability of recombinant human parathyroid hormone (rhPTH[1–84]) in a randomized, double-blind, placebo-controlled study for the treatment of adults with hypoparathyroidism. In: The Endocrine Society’s 94th Annual Meeting and Expo. Houston, TX SUN-325: 2012.
Bilezikian JP, Clarke B, Mannstadt M, et al. Effect of recombinant human parathyroid hormone (rhPTH[1–84]) on skeletal dynamics and BMD in hypoparathyroidism: the REPLACE study. In: Program of the 34th Annual Meeting of the American Society of Bone and Mineral Research. Minneapolis, MN MO0132: 2012.
Hattersley G, Bilezikian J, Guerriero J, et al. Bone anabolic efficacy and safety of BA058, a novel analog of hPTHrP: results from a phase 2 clinical trial in postmenopausal women with osteoporosis. In: The Endocrine Society’s 94th Annual Meeting and Expo. Houston, TXOR08-1: 2012.
Cusano NE, Rubin MR, McMahon DJ, et al. Therapy of hypoparathyroidism with PTH(1–84): a prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab 2013, 98: 137–44.
Lindsay R, Nieves J, Henneman E, et al. Subcutaneous administration of the amino-terminal fragment of human parathyroid hormone-(1–34): kinetics and biochemical response in estrogenized osteoporotic patients. J Clin Endocrinol Metab 1993, 77: 1535–9.
Schwietert HR, Groen EW, Sollie FA, Jonkman JH. Single-dose subcutaneous administration of recombinant human parathyroid hormone [rhPTH(1–84)] in healthy postmenopausal volunteers. Clin Pharmacol Ther 1997, 61: 360–76.
Rubin MR, Bilezikian JP. Hypoparathyroidism: clinical features, skeletal microstructure and parathyroid hormone replacement. Arq Bras Endocrinol Metabol 2010, 54: 220–6.
Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol 2002, 30: 312–21.
Jolette J, Wilker CE, Smith SY, et al. Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1–84 in a 2-year study in Fischer 344 rats. Toxicol Pathol 2006, 34: 929–40.
Andrews EB, Gilsenan AW, Midkiff K, et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: Study design and findings from the first 7 years. J Bone Miner Res 2012, 27: 2429–37.
Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res 2012, 27: 2419–28.
Diaz-Soto G, Mora-Porta M, Nicolau J, et al. Efficacy and safety of long term treatment of unresponsive hypoparathyroidism using multipulse subcutaneous infusion of teriparatide. Horm Metab Res 2012, 44: 708–10.
Theman TA, Collins MT, Dempster DW, et al. PTH(1–34) replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J Bone Miner Res 2009, 24: 964–73.
Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1–34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res 2011, 28: 159–65.
Hattersley G, Bilezikian JP, Guerriero J et al. Bone anabolic efficacy and safety of BA058, a novel analog of hPTHrP: results from a phase 2 clinical trial in postmenopausal women with osteoporosis. In: The Endocrine Society’s 94th Annual Meeting and Expo. Houston, TX OR08-1: 2012
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cusano, N.E., Rubin, M.R., Irani, D. et al. Use of parathyroid hormone in hypoparathyroidism. J Endocrinol Invest 36, 1121–1127 (2013). https://doi.org/10.1007/BF03346763
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03346763