Summary
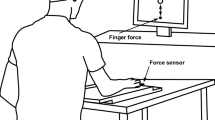
The present report examines the control strategy adopted by subjects to modulate the amplitude of transient force responses aimed to a target. Previous studies (Freund and Budingen 1978; Ghez and Vicario 1978) suggest that subjects modulate the rate of rise of force while maintaining force rise time at a near-constant value, independent of peak force. Such studies, however, have examined only the most rapid responses where force rise time could have been at a physiological limit. We now examine whether this control policy is dependent on an instruction to produce the fastest possible trajectories, or whether it is freely selected by subjects to maximize accuracy when rise time is unconstrained. We compared responses made by six subjects, under two task conditions: 1. Fast, “make the force impulse as brief as possible”; and 2. Accurate, “be as accurate as possible without regard to rise time”. Subjects were trained to produce monotonic flexion force impulses at the elbow to match the amplitudes of visually presented target shifts. Targets of three different were presented in randomized order. Responses made under the Accurate condition were less variable at each target amplitude than those under the Fast condition. Under both conditions, the initial peaks of the first and second time derivatives of force, early measures of trajectory dynamics, were strongly predictive of the peak force achieved and were correlated with the required force (target amplitude). Therefore, response trajectories must have been largely preprogrammed, and, further, the degree to which the initial peak d2F/dt2 predicts the peak force achieved represents a measure of the contribution of a preplanned motor program to trajectory formation. Subjects showed two systematic differences in trajectories between conditions. First, in all subjects force rise time was greater in the Accurate condition than in the Fast condition. Second, while in the Fast condition there was a modest dependence of force rise time on peak force, in the Accurate condition this dependence disappeared. Thus, when subjects were attempting to be as accurate as possible, they more consistently regulated force rise time around a constant value. This pulse height control policy allows responses of different amplitudes to be produced by proportional scaling of a stereotyped waveform. We conclude that a pulse height control policy with regulation of force rise time is a strategy adopted by subjects to simplify accurate control of response amplitude.
Similar content being viewed by others
References
Arbib MA (1981) Perceptual structures and distributed motor control. In: Brooks VB (ed) Handbook of physiology, Sect 1. The nervous system, Vol 2. Motor control. American Physiological Society, Bethesda, pp 1449–1480
Bahill AT, Clark MR, Stark L (1975) The main sequence, a tool for studying eye movements. Math Biosci 24: 191–204
Bernstein NA (1967) The coordination and regulation of movements. Pergamon Press, New York
Bouisset S, Lestienne F (1974) The organization of a simple voluntary movement as analyzed from its kinematic properties. Brain Res 71: 451–457
Brooks VB (1974) Some examples of programmed limb movements. Brain Res 71: 299–308
Brooks VB (1979) Motor programs revisited. In: Talbott RE, Humphrey DR (eds) Posture and movement. Raven Press, New York, pp 13–49
Burke RE, Rudomin P, Zajac FE (1970) Catch property in single mammalian motor units. Science 168: 122–124
Crossman ERFW, Goodeve PJ (1963) Feedback control of handmovement and Fitts' Law. Proceedings of the Experimental Psychology Society, Oxford 1963. Published (1983) in Q J Exp Psychol 35A: 251–278
Desmedt JE, Godaux E (1977) Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol 264: 673–693
Desmedt JE, Godaux E (1978) Ballistic skilled movements: load compensation and patterning of the motor commands. In: Desmedt JE (ed) Progress in clinical neurophysiology, Vol 4. Cerebral motor control in man: long loop mechanisms. Karger, Basel, pp 21–55
Enoka RM (1983) Muscular control of a learned movement: the speed control system hypothesis. Exp Brain Res 51: 135–145
Evarts EV (1971) Feedback and corollary discharge: a merging of the concepts. Neurosci Res Prog Bull 9(1): 86–112
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391
Freund H-J, Büdingen HJ (1978) The relationship between speed and amplitude of the fastest voluntary contractions of human arm muscles. Exp Brain Res 31: 1–12
Georgopoulos AP, Kalaska JP, Massey JT (1981) Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophysiol 46: 725–743
Ghez C (1979) Contributions of central programs to rapid limb movement in the cat. In: Asanuma H, Wilson V (eds) Integration in the nervous system. Igaku-Shoin, Tokyo, pp 305–319
Ghez C, Gordon J (1987) Trajectory control in targeted force impulses. I. Role of opposing muscles. Exp Brain Res 67: 225–240
Ghez C, Vicario D (1978) The control of rapid limb movement in the cat. II. Scaling of isometric force adjustments. Exp Brain Res 33: 191–202
Ghez C, Vicario D, Martin JH, Yumiya H (1983) Sensory motor processing of targeted movements in motor cortex. In: Desmedt JE (ed) Motor control mechanisms in health and disease. Raven Press, New York, pp 61–92
Gordon J (1985) Mechanisms contributing to accuracy in aimed force impulses of human subjects. Doctoral dissertation. Columbia University, Teachers College
Gordon J, Ghez C (1984) EMG patterns in antagonist muscles during isometric contraction in man: relations to response dynamics. Exp Brain Res 55: 167–171
Gordon J, Ghez C (1985) Mechanisms contributing to accuracy in aimed force impulses: rise time regulation and corrective adjustments. Soc Neurosci Abstr 11: 75
Gordon J, Ghez C (1987) Trajectory control in targeted force impulses. III. Compensatory adjustments for initial errors. Exp Brain Res 67: 253–269
Greene PH (1972) Problems of organization of motor systems. In: Rosen R, Snell FM (eds) Progress in theoretical biology. Academic Press, New York, pp 303–338
Grillner S (1975) Locomotion in vertebrates: Central mechanisms and reflex interaction. Physiol Rev 55: 247–304
Gurfinkel VS, Levik YS (1973) Dependence of contraction of the muscle on the sequence of stimulating pulses. Biophysics (USSR) 18: 116–121
Hallett M, Shahani BT, Young RR (1975) EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry 38: 1154–1162
Higgins JR, Angel RW (1970) Correction of tracking errors without sensory feedback. J Exp Psychol 84: 412–416
Hollerbach JM, Flash T (1982) Dynamic interactions between limb segments during planar arm movement. Biol Cybern 44: 67–77
Howarth CI, Beggs WDA, Bowden JM (1971) The relationship between speed and accuracy of movement aimed at a target. Acta Psychol 35: 207–218
Keele SW (1968) Movement control in skilled motor performance. Psychol Bull 70: 387–403
Kornhuber HH (1971) Motor functions of cerebellum and basal ganglia: the cerebellocortical saccadic (ballistic) clock, the cerebellonuclear hold regulator, and the basal ganglia ramp (voluntary speed smooth movement) generator. Kybernetic 8: 157–162
Laurutis VP, Robinson DA (1986) The vestibular-ocular reflex during human saccadic eye movements. J Physiol 373: 208–233
Nashner LM (1979) Organization and programming of motor activity during posture control. Progr Brain Res 50: 177–184
Oscarsson O (1973) Functional organization of spinocerebellar paths. In: Iggo A (ed) Somatosensory system. Handbook of sensory physiology, Vol II Springer, Berlin Heidelberg New York, pp 339–380
Pew RW (1970) Toward a process-oriented theory of human skilled performance. J Mot Behav 2: 8–24
Schmidt RA, Zelaznik H, Hawkins B, Frank JS, Quinn JT (1979) Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol Rev 86: 415–451
Soechting JF, Lacquaniti F (1981) Invariant characteristics of a pointing movement in man. J Neurosci 1: 710–720
Stein RB, Parmiggiani F (1979) Optimal motor patterns for activating mammalian muscle. Brain Res 175: 372–376
Stetson RH (1905) A motor theory of rhythm and discrete succession. Psychol Rev 12: 250–271
Stetson RH, McDill JA (1923) Mechanisms of the different types of movements. Psychol Med (Monogr Suppl) 32: 18–40
Taylor FV, Birmingham HP (1948) Studies of tracking behavior. II. The acceleration pattern of quick manual corrective responses. J Exp Psychol 38: 783–795
Vicario DS, Ghez C (1984) The control of rapid limb movement in the cat. IV. Updating of ongoing isometric responses. Exp Brain Res 55: 134–144
Viviani P, McCollum G (1983) The relation between linear extent and velocity in drawing movements. Neurosci 10: 211–218
Woodworth RS (1899) The accuracy of voluntary movement. Psychol Rev 3 (2, Suppl 13): 1–114
Zajac FE, Young JL (1976) Discharge patterns of motor units during cat locomotion and their relation to muscle performance. In: Herman RM, Grillner S, Stein PSG, Stuart DG (eds) Neural control of locomotion. Plenum Press, New York, pp 789–793
Zee DS, Optican LM, Cook JD, Robinson DA, Engel WK (1976) Slow saccades in spinocerebellar degeneration. Arch Neurol 33: 243–251
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gordon, J., Ghez, C. Trajectory control in targeted force impulses. Exp Brain Res 67, 241–252 (1987). https://doi.org/10.1007/BF00248546
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248546