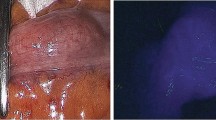
Abstract
Background
Anastomotic leakage (AL) is one of the dreaded complications following surgery in the digestive tract. Near-infrared fluorescence (NIRF) imaging is a means to intraoperatively visualize anastomotic perfusion, facilitating fluorescence image-guided surgery (FIGS) with the purpose to reduce the incidence of AL. The aim of this study was to analyze the current practices and results of NIRF imaging of the anastomosis in digestive tract surgery through the EURO-FIGS registry.
Methods
Analysis of data prospectively collected by the registry members provided patient and procedural data along with the ICG dose, timing, and consequences of NIRF imaging. Among the included upper-GI, colorectal, and bariatric surgeries, subgroup analysis was performed to identify risk factors associated with complications.
Results
A total of 1240 patients were included in the study. The included patients, 74.8% of whom were operated on for cancer, originated from 8 European countries and 30 hospitals. A total of 54 surgeons performed the procedures. In 83.8% of cases, a pre-anastomotic ICG dose was administered, and in 60.1% of cases, a post-anastomotic ICG dose was administered. A significant difference (p < 0.001) was found in the ICG dose given in the four pathology groups registered (range: 0.013–0.89 mg/kg) and a significant (p < 0.001) negative correlation was found between the ICG dose and BMI. In 27.3% of the procedures, the choice of the anastomotic level was guided by means of NIRF imaging which means that in these cases NIRF imaging changed the level of anastomosis which was first decided based on visual findings in conventional white light imaging. In 98.7% of the procedures, the use of ICG partly or strongly provided a sense of confidence about the anastomosis. A total of 133 complications occurred, without any statistical significance in the incidence of complications in the anastomoses, whether they were ICG-guided or not.
Conclusion
The EURO-FIGS registry provides an insight into the current clinical practice across Europe with respect to NIRF imaging of anastomotic perfusion during digestive tract surgery.


Similar content being viewed by others
References
Kassis ES, Kosinski AS, Ross P Jr, Koppes KE, Donahue JM, Daniel VC (2013) Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 96(6):1919–1926
Biere SS, Maas KW, Cuesta MA, van der Peet DL (2011) Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 28(1):29–35
Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246(3):363–72; discussion 72–4.
Lang H, Piso P, Stukenborg C, Raab R, Jahne J (2000) Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol 26(2):168–171
Haga Y, Wada Y, Takeuchi H, Ikejiri K, Ikenaga M (2011) Prediction of anastomotic leak and its prognosis in digestive surgery. World J Surg 35(4):716–722
Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K (2015) Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol 21(32):9656–9665
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102(5):462–479
European Society of Coloproctology Collaborating G (2018) The 2017 European Society of Coloproctology (ESCP) international snapshot audit of left colon, sigmoid and rectal resections—executive summary. Colorectal Dis 20(6):13–41
Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, Carmichael JC et al (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148(1):65–71
Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY et al (2013) Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 257(4):665–671
Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P (2008) Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 23(3):265–270
Midura EF, Hanseman D, Davis BR, Atkinson SJ, Abbott DE, Shah SA et al (2015) Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum 58(3):333–338
Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A et al (2015) Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 220(2):195–206
Pommergaard HC, Achiam MP, Burcharth J, Rosenberg J (2015) Impaired blood supply in the colonic anastomosis in mice compromises healing. Int Surg 100(1):70–76
Karliczek A, Benaron DA, Zeebregts CJ, Wiggers T, van Dam GM (2009) Intraoperative ischemia of the distal end of colon anastomoses as detected with visible light spectroscopy causes reduction of anastomotic strength. J Surg Res 152(2):288–295
Trencheva K, Morrissey KP, Wells M, Mancuso CA, Lee SW, Sonoda T et al (2013) Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann Surg 257(1):108–113
van den Bos J, Al-Taher M, Schols RM, van Kuijk S, Bouvy ND, Stassen LPS (2018) Near-infrared fluorescence imaging for real-time intraoperative guidance in anastomotic colorectal surgery: a systematic review of literature. J Laparoendosc Adv Surg Tech A 28(2):157–167
Blanco-Colino R, Espin-Basany E (2018) Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 22(1):15–23
Shen R, Zhang Y, Wang T (2018) Indocyanine green fluorescence angiography and the incidence of anastomotic leak after colorectal resection for colorectal cancer: a meta-analysis. Dis Colon Rectum 61(10):1228–1234
Agnus V, Pesce A, Boni L, Van Den Bos J, Morales-Conde S, Paganini AM et al (2019) Fluorescence-based cholangiography: preliminary results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg Endosc. 34:3888–3896
Gero D, Gie O, Hubner M, Demartines N, Hahnloser D (2017) Postoperative ileus: in search of an international consensus on definition, diagnosis, and treatment. Langenbecks Arch Surg 402(1):149–158
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24(5):569–576
Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA et al (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 220(1):82-92 el
Zehetner J, DeMeester SR, Alicuben ET, Oh DS, Lipham JC, Hagen JA et al (2015) Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after esophagectomy. Ann Surg 262(1):74–78
Noma K, Shirakawa Y, Kanaya N, Okada T, Maeda N, Ninomiya T et al (2018) Visualized evaluation of blood flow to the gastric conduit and complications in esophageal reconstruction. J Am Coll Surg 226(3):241–251
Van Daele E, Van Nieuwenhove Y, Ceelen W, Vanhove C, Braeckman BP, Hoorens A et al (2019) Near-infrared fluorescence guided esophageal reconstructive surgery: a systematic review. World J Gastrointest Oncol 11(3):250–263
Kumagai Y, Hatano S, Sobajima J, Ishiguro T, Fukuchi M, Ishibashi KI et al (2018) Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus. https://doi.org/10.1093/dote/doy052
Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI et al (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102(2):e169–e176
Diana M, Dallemagne B, Chung H, Nagao Y, Halvax P, Agnus V et al (2014) Probe-based confocal laser endomicroscopy and fluorescence-based enhanced reality for real-time assessment of intestinal microcirculation in a porcine model of sigmoid ischemia. Surg Endosc 28(11):3224–3233
Diana M, Halvax P, Dallemagne B, Nagao Y, Diemunsch P, Charles AL et al (2014) Real-time navigation by fluorescence-based enhanced reality for precise estimation of future anastomotic site in digestive surgery. Surg Endosc 28(11):3108–3118
Diana M, Noll E, Agnus V, Liu YY, Kong SH, Legner A et al (2017) Reply to letter: “Enhanced Reality Fluorescence Videography to Assess Bowel Perfusion: The Cybernetic Eye.” Ann Surg 265(4):e49–e52
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V et al (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259(4):700–707
Hayami S, Matsuda K, Iwamoto H, Ueno M, Kawai M, Hirono S et al (2019) Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech Coloproctol 23(10):973–980
Seeliger B, Agnus V, Mascagni P, Barberio M, Longo F, Lapergola A et al (2020) Simultaneous computer-assisted assessment of mucosal and serosal perfusion in a model of segmental colonic ischemia. Surg Endosc 34(11):4818–4827
Iwamoto H, Matsuda K, Hayami S, Tamura K, Mitani Y, Mizumoto Y et al (2020) Quantitative indocyanine green fluorescence imaging used to predict anastomotic leakage focused on rectal stump during laparoscopic anterior resection. J Laparoendosc Adv Surg Tech A 30(5):542–546
D’Urso A, Agnus V, Barberio M, Seeliger B, Marchegiani F, Charles AL et al (2020) Computer-assisted quantification and visualization of bowel perfusion using fluorescence-based enhanced reality in left-sided colonic resections. Surg Endosc. https://doi.org/10.1007/s00464-020-07922-9
Foppa C, Denoya PI, Tarta C, Bergamaschi R (2014) Indocyanine green fluorescent dye during bowel surgery: are the blood supply “guessing days” over? Tech Coloproctol 18(8):753–758
Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S et al (2015) Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 29(7):2046–2055
Benya R, Quintana J, Brundage B (1989) Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn 17(4):231–233
Acknowledgments
The authors would like to thank: Drs Michela Scollica, Amedeo Elio, and Sergi Sanchez Cordero for their contribution in collecting data and Guy Temporal and Christopher Burel, professionals in medical English proofreading, for their valuable assistance.
Funding
The EURO-FIGS registry is funded by a grant from the ARC Foundation for Cancer Research (9, rue Guy Môquet; 94803 Villejuif Cedex, France, www.fondation-arc.org), within the framework of the ELIOS (Endoscopic Luminescent Imaging for precision Oncologic Surgery) project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Michele Diana is the PI and the recipient of the ELIOS grant from the ARC foundation and is member of the Advisory Board of Diagnostic Green. Salvador Morales Conde reports grants and other relationships with Medtronic and other relationships with BD Bard, Ethicon, Olympus, Storz, Stryker, Dipro, Baxter, and BBraum, outside the submitted work. Gianluca Baiocchi reports paid consultation for Stryker corp and travel grant from Karl Storz and from Stryker corp. Luigi Boni played a role as consultant for company producing fluorescent-guided surgery devices. Laurents Stassen reports other relationships with Diagnostic Green, outside the submitted work. Jacques Marescaux is the President of the IRCAD, which is partly funded by KARL STORZ and Medtronic. Andrea Spota, Mahdi Al-Taher, Eric Felli, Ivano Dal Dosso, Gianluigi Moretto, Giuseppe Spinoglio, Ramon Vilallonga, Harmony Impellizzeri, Gonzalo P. Martin-Martin, Lorenzo Casali, Christian Franzini, Marta Silvestri, Nicolò de Manzini, Maurizio Castagnola, Marco Filauro, Davide Cosola, Catalin Copaescu, Giovanni Maria Garbarino, Antonio Pesce, Marcello Calabrò, Paola De Nardi, Gabriele Anania, Thomas Carus, Alessandro Patanè, Caterina Santi, Alend Saadi, Alessio Rollo, Roland Chautems, José Noguera, Jan Grosek, Giancarlo D’Ambrosio, Carlos Marques Ferreira, Gregor Norcic, Giuseppe Navarra, Pietro Riva, Silvia Quaresima, Alessandro Paganini, Nunzio Rosso, Paolo De Paolis, Andrea Balla, Marc-Olivier Sauvain, Eleftherios Gialamas, Giorgio Bianchi, Gaetano La Greca, Carlo Castoro, Andrea Picchetto, Alessandro Franchello, Luciano Tartamella, Robert Juvan, Orestis Ioannidis, Jurij Ales Kosir, and Emilio Bertani have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A: List of items registered
Appendix A: List of items registered
-
Patient age.
-
Patient gender.
-
Patient BMI.
-
Patient comorbidities.
-
Diagnosis requiring surgery.
-
Neoadjuvant radiotherapy?
-
Neoadjuvant chemotherapy?
-
Surgical procedure performed.
-
Type of anastomosis.
-
Near-infrared camera model.
-
Evaluation of anastomotic perfusion?
-
ICG dose (mg/kg).
-
Pre-anastomotic ICG injection?
-
Reinjection?
-
Post-anastomotic ICG injection?
-
Adverse events of ICG administration?
-
Did ICG influence the transection line?
-
Did ICG provide you with a sense of confidence concerning the perfusion of your anastomosis?
-
Did your patient present any clinical sign of post-operative complications?
-
Did your patient need any post-operative radiological investigation?
-
Do you have any other comment?
Rights and permissions
About this article
Cite this article
Spota, A., Al-Taher, M., Felli, E. et al. Fluorescence‐based bowel anastomosis perfusion evaluation: results from the IHU‐IRCAD‐EAES EURO‐FIGS registry. Surg Endosc 35, 7142–7153 (2021). https://doi.org/10.1007/s00464-020-08234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08234-8