Abstract
Aims
The role of laparoscopy in rectal cancer has been questioned. 3D laparoscopic systems are suggested to aid optimal surgical performance but have not been evaluated in advanced procedures. We hypothesised that stereoscopic imaging could improve the performance of laparoscopic total mesorectal excision (TME).
Methods
A multicentre developmental randomised controlled trial comparing 2D and 3D laparoscopic TME was performed (ISRCTN59485808). Trial surgeons were colorectal consultants that had completed their TME proficiency curve and underwent stereoscopic visual testing. Patients requiring elective laparoscopic TME with curative intent were centrally randomised (1:1) to 2D or 3D using Karl Storz IMAGE1 S D3-Link™ and 10-mm TIPCAM®1S 3D passive polarising laparoscopic systems. Outcomes were enacted adverse events as assessed by the observational clinical human reliability analysis technique, intraoperative data, 30-day patient outcomes, histopathological specimen assessment and surgeon cognitive load.
Results
88 patients were included. There were no differences in patient or tumour demographics, surgeon stereopsis, case difficulty, cognitive load, operative time, blood loss or conversion between the trial arms. 1377 intraoperative adverse events were identified (median 18 per case, IQR 14–21, range 2–49) with no differences seen between the 2D and 3D arms (18 (95% CI 17–21) vs. 17 (95% CI 16–19), p = 0.437). 3D laparoscopy had non-significantly higher mesorectal fascial plane resections (94 vs. 77%, p = 0.059; OR 0.23 (95% CI 0.05–1.16)) but equal lymph node yield and circumferential margin distance and involvement. 30-day morbidity, anastomotic leak, re-operation, length of stay and readmission rates were equal between the 2D and 3D arms.
Conclusion
Feasibility of performing multicentre 3D laparoscopic multicentre trials of specialist performed complex procedures is shown. 3D imaging did not alter the number of intraoperative adverse events; however, a potential improvement in mesorectal specimen quality was observed and should form the focus of future 3D laparoscopic TME trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The role of minimal access surgery (MAS) in total mesorectal excision (TME) is hotly contested. Oncological outcomes are closely linked to the technical performance of surgery, specifically through the quality of the TME specimen [1,2,3,4,5]. Medium-term follow-up of multicentre randomised controlled trials (RCTs) suggest that laparoscopic rectal surgery can be performed without oncological compromise [6,7,8]; however, two recent large RCTs showed that although the majority of laparoscopic cases had acceptable specimens, laparoscopic non-inferiority could not be shown [9, 10]. This topic is highly pertinent as because of perceived short-term patient benefits 68% of UK rectal cancer patients presently receive a laparoscopic operation [7, 11, 12].
The MAS revolution is facilitated by continuous technological development. Advances in laparoscopic platforms include commercially available three-dimensional (3D) HD systems. Initial adoption was hampered by poor image resolution and bulky headgear associated with unacceptable user side effects [13]. Modern refinement of 3D technology has revived surgical interest as contemporary systems have overcome these issues without increasing cognitive load [14,15,16].
The potential advantages of 3D imaging systems on the performance or outcomes following advanced laparoscopic procedures have not been proved as the available literature predominantly focusses on trainee performance of ex-vivo box trainer tasks with significant methodological concerns raised [14, 16, 17]. Therefore, we designed a development trial with the dual aims of comparing specialist surgical performance of laparoscopic TME surgery using 2D and 3D imaging and to generate evidence to identify and power the appropriate primary endpoint for use in a future definitive TME study.
Methods
A four-centre, parallel arm (1:1), stage 2b exploration study developmental randomised controlled trial was designed in keeping with the IDEAL recommendations as well as quality assurance in multicentre laparoscopic colorectal trials, 3D laparoscopic studies and CONSORT principles [14, 17,18,19]. Ethical approval was granted by the UK National Health Service South Central - Berkshire B research ethics committee (16/SC/0118). This trial is registered (ISRCTN59485808).
Patient eligibility criteria
Study inclusion criteria were biopsy-proven adenocarcinoma of the rectum, ≤ 15 cm from the anal verge, age 18 ≤, provision of written informed consent and the responsible colorectal multi-disciplinary team advised elective laparoscopic TME undertaken with curative intent. Neoadjuvant chemoradiotherapy use remained at the discretion of the responsible clinicians. All patients were required to undergo minimum staging of pelvic MRI, CT chest, abdomen and pelvis, tumour biopsy and full colonic assessment with either optical colonoscopy or CT colonography. Exclusion criteria were known or suspected inflammatory bowel disease, emergency, unplanned or palliative surgery, locally advanced cancers (T4a—TNM 5th edition), refusal or inability to provide informed consent and concurrent or past abdominal or pelvic malignancy. Abdominal-perineal excisions, trans-anal TME and procedures where no anastomosis was planned were also excluded.
Surgeon eligibility criteria and stereopsis testing
Established experienced minimally invasive rectal cancer centres were approached to participate. All trial surgeons were required to have exceeded previously defined proficiency curve estimates and/or completed the UK LapCo consultant training programme as participant or tutor [20]. Surgeons took the Netherlands organisation for applied scientific research (TNO) stereoscopic visual test (19th edition, Laméris Ootech BV, Utrecht, The Netherlands). Participant stereo acuity was defined as the last correctly reported image with ≤ 120 s of arc considered normal.
Developmental endpoints and sample size
There was no prior 3D TME research to guide sample size calculations. To assess the impact of stereoscopic imaging on TME performance, the primary endpoint of this study was the total number of enacted intraoperative adverse events per case identified using the observational clinical human reliability analysis (OCHRA) methodology. In previous work, using a combination of open and 2D laparoscopic TME cases, we observed an average of 17 errors (± 7.02 [21]) with differences in specialist performances identified [22]. Using a 5% significant level, a sample size of 62 had 80% power to detect a decrease in error counts to 12. This minimally relevant 30% difference was chosen based on the difference in operative performance of laparoscopic colectomy in the UK LapCo national training programme sign off data as an estimate [22]. Allowing a 15% attrition rate for conversions or loss to follow-up the recruitment target was 72.
Clinical outcomes
Pre-defined secondary endpoints were operative factors (time, blood loss, stoma creation and conversion—defined as inability to complete the dissection including the vascular ligation and/or requiring an incision larger than that needed for specimen extraction), histopathologically assessed specimen quality (plane of mesorectal excision, lymph node yield, circumferential resection margin and complete excision [2]) and 30-day patient outcomes morbidity (using the Clavien–Dindo classification [23], length of stay and unplanned reattendance or readmission to hospital). As 3D systems have the potential to influence surgeon cognitive load, the NASA-task load index (NASA-TLX) was completed following each case [24]. This widely applied and previously validated surgeon reported system represents the most commonly used measurement method to assess cognition in the operating theatre setting [25, 26].
Observational clinical human reliability analysis (OCHRA)
To assess whether 3D imaging influenced surgical performance, assessment of the intraoperative period is required to provide detailed analysis of the intervention delivery. The OCHRA technique was adopted in keeping with previous descriptions used for the assessment of specialist performance of laparoscopic colorectal resections and the primary endpoint of a multicentre TME RCT [21, 22, 27]. Briefly, OCHRA involves structured analysis of unedited case video to identify adverse events defined as “something that was not intended by the surgeon, nor desired by a set of rules or an external observer, or that led the task outside acceptable limits” [28]. Events were further categorised by instrument used, external error mode, instrument/dissection or tissue/retraction errors (based upon the perceived principal mechanism for the event) and any resulting consequence used previously reported pre-defined coding lists (Table 2 and Table 4). Errors occur across all task phases not just the pelvis [21, 22, 27], therefore analysis of the entire case was performed. Operative phase of surgery was also captured using a hierarchical task analysis based upon an international consensus [21, 29]. Deviation from this order was not considered as an error. Video review was performed after OCHRA training including blinded analysis of 20 previously recorded 2D laparoscopic TME cases with excellent inter-rater reliability observed (Intraclass correlation co-efficient 0.916).
Equipment, setup and procedures
All cases were performed using Karl Storz IMAGE1 S D3-Link™ laparoscopic systems with zero or 30° 10-mm TIPCAM®1 SPIES 3D video laparoscopes. Images were displayed on 32-inch LCD HD screens (model EJ-MDA32E-K) and viewed with passive polarising glasses (Panasonic® Europe, Wiesbaden, Germany). To minimise cross-talk and facilitate optimal viewing and ergonomic positioning, precise screen location and viewing distance was at the discretion of each surgical team. All participating surgeons stated that their usual operative plan matched the previously reported international TME standardisation report [29]. To maximise recruitment, generalisability of results and ethical and surgeon acceptability, no constraints on timing of surgery, operative technique, task order, instrument use or any on table decision were made. All perioperative care proceeded as per local site policies.
Data collection
Video recording utilised the integrated advanced image and data acquisition system (AIDA™, Karl Storz Endoskopy GmBH, Tuttlingen, Germany). Entire cases were recorded unedited in 2D irrespective of randomisation result, deidentified and labelled with a unique study ID as sole identifier. Immediately following case completion, surgeons completed the NASA-TLX instrument and a series of 100-mm visual analogue scales capturing overall case, task and pelvic complexity. Specimen analysis was performed at each site by specialist histopathologists blinded to trial arm and in keeping with the UK Royal College of Pathologists reporting dataset including a three-point ordinal scale for plane of mesorectal dissection. Patients were prospectively followed for 30 days by dedicated research staff independent of the trial. All complications were categorised using the Clavien–Dindo classification [30]. Video files were transferred to the central trial office for analysis using portable hard drives (Canvio Basics, Toshiba Europe, Weybridge, UK). Here, a second coding took place to further ensure blinded analysis.
Randomisation procedure
To ensure allocation concealment, upon recruitment, patients were randomised centrally to the 2D or 3D arms using a pre-defined computer-generated random number list. Given the sample size, no stratification was undertaken.
Statistical analyses
The data were analysed using SPSS (v24.0; SPSS Inc, Chicago, IL, USA). All data were explored for normality with the Shapiro–Wilk test and detrended Q–Q plots and compared with parametric or non-parametric tests as appropriate. t-test, Mann–Whitney U and Kruskal Wallis testing were used to compare medians from normal and non-normally distributed populations. For categorical data, analysis included the use of cross tabulation, Fisher’s exact test or chi-squared to test association between groups. Effect magnitude was quantified using odds ratio (OR) and 95% confidence intervals. Data are displayed as medians with interquartile ranges (IQR) unless specified. Comparative results are reported as (2D vs.3D) throughout. Analyses are reported as intention to treat except those solely based upon video analysis where the necessity for a complete case recording required a per protocol approach. Statistical significance was defined as p < 0.05.
Results
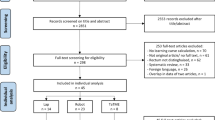
88 patients from four sites were randomised between June 2016 and March 2018 (Fig. 1). 58% were male. Average age, body mass index and tumour height from the anal verge were 69, 28 and 8.5 cm, respectively. 23% underwent neoadjuvant chemoradiotherapy. All patient and tumour demographics were evenly distributed (Table 1). Nine surgeons participated with no evidence of impaired stereo acuity (range 60–15 s of arc).
Operative data and surgeon reported case complexity
No differences were seen in surgeon reported overall case complexity (28 mm (IQR 18–43) vs. 31 mm (19–63), p = 0.399), any surgical phase or pelvic quadrants between the trial arms (Table 2). No differences in surgical time (278 (95% CI 270–360) vs. 270 min (235–335), p = 0.34), blood loss (60 vs. 90 ml, p = 0.618), conversion (2 (4.9%) vs. 2 (4.8%), p = 0.981), defunctioning ileostomy creation (89% vs. 85%, p = 0.587) or anastomosis height (3 vs. 3 cm, p = 0.829) were seen.
Short-term patient outcomes
A total of 110 morbidity events from 52 patients were recorded in the first 30 post-operative days (any morbidity 61.2%, median 1 per patient, IQR 0–2, range 0–5, Table 3) with no difference between trial arms (59.5% vs. 62.7%, odds ratio 1.2 (95% CI 0.5–2.9), p = 0.834) or Clavien–Dindo classification (p = 0.899). Anastomotic leak rate (overall 5.9%, 4.8% vs. 7%, p = 0.666) and re-operation rate (7.1% vs. 4.7%, p = 0.666) were comparable between the arms. Non-significant differences in length of hospital stay (9 (IQR 6–18) vs. 7 (5–15) days, p = 0.203) and re-admissions were observed (11.9% vs. 25.6%, p = 0.109).
OCHRA analysis
77 cases were analysed comprising 380 h of surgery. A total of 1377 intraoperative errors were identified (median 18 per case, IQR 14–21, range 2–49). No differences were seen between the 2D and 3D arms (18 (IQR 14–21) vs. 17 (IQR 13–22), p = 0.437). OCHRA categorical data are displayed in Fig. 2A–C and Table 4. Apart from a reduction in overshoot errors in 3D surgery (64 vs. 48, p = 0.05), no differences are seen in the data. Errors took place across all operative phases with 689 (50%, Fig. 2) taking place during pelvic tasks; however, no difference between the trial arms was seen (total 322 vs. 367, median 8 per case (6–12) vs. 8 (6–11), p = 0.854) or by pelvic location (Supplementary Table 1 + Supplementary Fig. 1).
A–C Intraoperative error data. A Box and whisker plot, B histogram, C errors per operative phase. No differences in the distributions are seen. Errors were seen to take place across all phases of the operation justifying the approach to review entire cases. Studying pelvic performance alone would have missed 50% of identified adverse events
Surgeon cognitive load
Surgeons reported low demands across all six domains of the NASA-TLX with no statistical or clinically relevant differences seen between the trial arms (Fig. 3).
Specimen analysis
Pathologically assessed tumour stages, relationship to the peritoneal reflection, lymph node yield and circumferential resection margins were equal between 2D and 3D surgery (Table 5). A single R1 resection was observed in each arm (p = 0.987). Intention-to-treat analysis showed no difference in mesorectal fascial plane surgery (76% vs. 81%, OR 0.73 (95% CI 0.26–2.08), p = 0.163). However, the plane was not reported in eight cases (9.4%) predominantly from 3D patients. When these were excluded, 3D laparoscopy produced clinically but not statistically significant higher rates of mesorectal plane excisions (77% vs. 94%, OR 0.23 (95% CI 0.05–1.16), p = 0.059, Fig. 4).
Histopathological assessment of the mesorectal surgical plane. Despite inclusion in the UK Royal College of Pathologists colorectal cancer dataset was not given in eight (9.4%) reports. When these are excluded a clinically significant increase in mesorectal fascial plane surgery is seen (87% overall, 77% vs. 94%, OR 0.23 (95% CI 0.05–1.16), p = 0.059)
Discussion
With the present debate on the role of MAS in rectal cancer surgery, appraisal of novel technology that may positively impact on outcomes is required. There has been an uptake in 3D laparoscopy in clinical settings despite little evidence to support its use. Since there was no prior research, and as advocated by the IDEAL collaboration on surgical innovation, it was important to perform a developmental study in order to assist the design a future definitive RCT [18]. Feasibility of the methodology and multicentre recruitment was also needed given the time and resource implications of major trials. Here, we incorporated all methodological recommendations for multicentre laparoscopic colorectal RCTs and 3D studies [14, 17, 19] and report the first TME trial using 3D laparoscopy.
Assisted by video capture technology integrated in most MAS platforms, we deliberately studied the frequently overlooked intraoperative period as it was felt this is where any impact of imaging technology was most likely to be seen. It was hoped this could provide new insights into trial findings and identify areas for targeted improvements. Using the validated, structured OCHRA technique which we previously successfully applied to the assessment of intraoperative specialist performance and as the primary endpoint of a multicentre TME RCT, provision of stereoscopic imaging did not alter the number of enacted error events. Although a margin of 30% was selected, the observed difference was nominal supporting our approach to perform this preliminary trial. Video review is hindered by its time-intensive nature and importantly did not link operative performance to specimen results. Therefore, its relevance is questionable and appears redundant in future TME studies.
Optimal oncological outcomes are obtained through achieving a complete TME resection including clear circumferential margins and mesorectal fascial plane surgery [1, 2, 4, 5, 31,32,33]. Our main finding was the potential improvement in TME specimen quality following 3D laparoscopy. No other differences were observed across any other outcome. 94% of 3D TME specimens were assessed as mesorectal fascial plane representing a clinically, but borderline statistically, significant improvement over 2D surgery. This figure exceeds the results reported by major laparoscopic rectal cancer trials including their open and robotic arms [6, 9, 10, 34]. Resection in the mesorectal fascial plane is associated with reduced local and distant recurrence and improvements in disease-free and overall survival. This result and the very low CRM involvement rate can be expected to lead to low rates of recurrence and together with the acceptable conversion, leak and re-operation rate support the ongoing use of laparoscopy by specialist surgeons. It should be noted that reflecting our exclusion of abdominal-perineal resections the average tumour height was slightly higher than the major trials and lower neoadjuvant use was seen in keeping with UK guidelines and practice.
Across all other pre-defined endpoints, equivalence between the 2D and 3D trials arms was seen. The equal operative, cognitive load and patient outcome data suggest specialist performance was not altered by the imaging technology used. It is possible their experience has overcome the lack of depth perception inherit to 2D laparoscopy. No meaningful surgeon side effects were encountered and no deterioration in cognitive load was seen suggesting contemporary 3D platforms have indeed overcome past deficiencies [13, 16]. Our results are strengthened by the use of centralised randomisation with allocation concealment, standardised equipment across all centres, stereopsis testing, blinded video assessment and independent histopathology and morbidity data collection.
Given the current literature concerns regarding laparoscopic TME specimen quality, our findings warrant further exploration. Mesorectal plane of excision should be adopted as the primary endpoint for a future larger multicentre RCT and would be additionally strengthened by the use of centralised, protocol-led specimen review. Our study design was agreeable to patients, surgeons and theatre teams resulting in acceptable recruitment with low attrition which should be reproducible across additional sites. Should a definitive study confirm our findings this would represent an easily implemented and generalisable route to quality improvement whilst delivering the short-term recovery benefits presented by MAS [7, 12]. Outside this endpoint, the equivalence of all other data does not support undertaking larger trials. To provide homogeneity, we excluded abdominal-perineal and trans-anal TME excisions. Although the need for a complete specimen is unaltered, variation in perineal and low rectal technique could have directly influenced histopathology results. The health economics of 3D laparoscopy have not been sufficiently reported to date although a recent health technology assessment suggested the additional cost per patient for 3D systems in general surgery could be as low as €1.67 [15]. Our data suggest no meaningful secondary impact on healthcare resources could be expected.
Surgical intervention research presents specific challenges but the need for evidence-based practice remains including in the use of theatre technologies [18]. It remains surprising that surgical technology undergoes intensive development and testing to obtain licencing but clinical research assessment is not mandatory. This is in direct contradiction to the extensive regulatory requirements for other healthcare interventions such as pharmaceuticals. The few randomised clinical 3D studies have also shown equivalent results going back over 20 years [35]. Randomisation removes many of the inherent biases that can unduly influence comparable studies. Our trial surgeons subjectively praised 3D systems and were surprised when data were unblinded in a similar fashion to other colorectal MAS technology trials [34, 36]. The majority of 3D laparoscopy studies have used box trainers and laparoscopically naïve participants limiting the applicability to OR performance [16, 17].
This study should be considered in view of its limitations. In nearly 10% of cases, no mesorectal plane assessment was given despite being a core requirement for TME histology reporting. These data may have influenced our conclusions, but early identification of this issue shows the strength of undertaking preliminary studies and will improve future RCT design. Although we successfully met our aims, as a developmental study with a modest sample size, firm conclusions should not be drawn. We complied with the CONSORT criteria however laparoscopic case selection bias cannot be fully excluded as pre-operative decision making and open TME surgery performed at each centre during the study timeframe were not captured. Although cognitive load was measured, case video does not capture human factors including team experience, interaction and distraction that could influence surgeon performance or the extracorporeal operative tasks. The 500 h of video analysis undertaken here highlights the limited applicability to routine clinical practice. Finally, the results obtained reflect the expertise of the participating surgeons and their centres and cannot be assumed to be applicable to trainees or inexperienced laparoscopic TME surgeons.
Conclusion
Feasibility of performing multicentre 3D laparoscopic multicentre trials of specialist performed complex procedures is shown. 3D imaging did not alter the number of intraoperative adverse events; however, a potential improvement in mesorectal specimen quality was observed and should form the focus of future 3D laparoscopic TME trials.
References
Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH (2002) Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 20(7):1729–1734
Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J et al (2009) Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 373(9666):821–828
Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1(8496):1479–1482
Leonard D, Penninckx F, Laenen A, Kartheuser A (2015) Scoring the quality of total mesorectal excision for the prediction of cancer-specific outcome. Colorectal Dis 17(5):O115–O122
Kitz J, Fokas E, Beissbarth T, Strobel P, Wittekind C, Hartmann A et al (2018) Association of plane of total mesorectal excision with prognosis of rectal cancer: secondary analysis of the CAO/ARO/AIO-04 phase 3 randomized clinical trial. JAMA Surg https://doi.org/10.1001/jamasurg.2018.1607
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, ES dL-dK et al (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372(14):1324–1332
Vennix S, Pelzers L, Bouvy N, Beets GL, Pierie JP, Wiggers T et al Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 2014(4):CD005200
Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB et al (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15(7):767–774
Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ et al (2015) Effect of laparoscopic-assisted resection versus open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314(13):1356–1363
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M et al (2015) Effect of laparoscopic-assisted resection versus open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314(13):1346–1355
(HQIP) THQIP. UK National Bowel Cancer Audit Annual Report 2017 6th July 2018
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW et al (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11(7):637–645
Schwab K, Smith R, Brown V, Whyte M, Jourdan I (2017) Evolution of stereoscopic imaging in surgery and recent advances. World J Gastrointest Endosc 9(8):368–377
Sakata S, Watson MO, Grove PM, Stevenson AR (2016) The conflicting evidence of three-dimensional displays in laparoscopy: a review of systems old and new. Ann Surg 263(2):234–239
Vettoretto N, Foglia E, Ferrario L, Arezzo A, Cirocchi R, Cocorullo G et al (2018) Why laparoscopists may opt for three-dimensional view: a summary of the full HTA report on 3D versus 2D laparoscopy by S.I.C.E. (Societa Italiana di Chirurgia Endoscopica e Nuove Tecnologie). Surg Endosc 32(6):2986–2993
Arezzo A, Vettoretto N, Francis NK, Bonino MA, Curtis NJ, Amparore D et al (2018) The use of 3D laparoscopic imaging systems in surgery: EAES consensus development conference 2018. Surg Endosc. https://doi.org/10.1007/s00464-018-06612-x
Sorensen SM, Savran MM, Konge L, Bjerrum F (2016) Three-dimensional versus two-dimensional vision in laparoscopy: a systematic review. Surg Endosc 30(1):11–23
McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC et al (2009) No surgical innovation without evaluation: the IDEAL recommendations. Lancet 374(9695):1105–1112
Foster JD, Mackenzie H, Nelson H, Hanna GB, Francis NK (2014) Methods of quality assurance in multicenter trials in laparoscopic colorectal surgery: a systematic review. Ann Surg 260(2):220–229
Coleman MG, Hanna GB, Kennedy R (2011) The National Training Programme for Laparoscopic Colorectal Surgery in England: a new training paradigm. Colorectal Dis 13(6):614–616
Foster JD, Miskovic D, Allison AS, Conti JA, Ockrim J, Cooper EJ et al (2016) Application of objective clinical human reliability analysis (OCHRA) in assessment of technical performance in laparoscopic rectal cancer surgery. Tech Coloproctol 20(6):361–367
Miskovic D, Ni M, Wyles SM, Parvaiz A, Hanna GB (2012) Observational clinical human reliability analysis (OCHRA) for competency assessment in laparoscopic colorectal surgery at the specialist level. Surg Endosc 26(3):796–803
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
SG H (1998) LE S. Development of NASA-TLX (Task load index): results of empirical and theoretical research. Adv Psychol 52:139–183
SG H. NASA-Task Load index (NASA-TLX); 20 years later. Proceedings of the Human Factors and Ergonomics Society Ergonomics Society Annual Meeting 2006; 1/1/20062006. p. 904-8
Dias RD, Ngo-Howard MC, Boskovski MT, Zenati MA, Yule SJ (2018) Systematic review of measurement tools to assess surgeons’ intraoperative cognitive workload. Br J Surg 105(5):491–501
Foster JD, Ewings P, Falk S, Cooper EJ, Roach H, West NP et al (2016) Surgical timing after chemoradiotherapy for rectal cancer, analysis of technique (STARRCAT): results of a feasibility multi-centre randomized controlled trial. Tech Coloproctol 20:683–693
Senders JW, Moray NP(1991) Human error: cause, prediction and reduction, 1 edn. CRC Press, Hillsdale, p 168
Miskovic D, Foster J, Agha A, Delaney CP, Francis N, Hasegawa H et al (2015) Standardization of laparoscopic total mesorectal excision for rectal cancer: a structured international expert consensus. Ann Surg 261(4):716–722
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Debove C, Maggiori L, Chau A, Kanso F, Ferron M, Panis Y (2015) What happens after R1 resection in patients undergoing laparoscopic total mesorectal excision for rectal cancer? a study in 333 consecutive patients. Colorectal Dis 17(3):197–204
Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA et al (2018) Disease-free survival and local recurrence for laparoscopic Resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg https://doi.org/10.1097/SLA.0000000000003002
Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD et al (2018) Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg https://doi.org/10.1097/SLA.0000000000003021
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J et al (2017) Effect of robotic-assisted versus conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for Rectal cancer: the ROLARR randomized clinical trial. JAMA 318(16):1569–1580
Hanna GB, Shimi SM, Cuschieri A (1998) Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet 351(9098):248–251
Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP (2012) Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 99(9):1219–1226
Acknowledgements
The authors gratefully acknowledge the support and contribution of the following in the delivery of this study: Nicky Marks, Tressy Pitt-Kerby, Lucy Pippard, Linda Howard, Christy Felix, Jake Foster, Steve Gore, Godwin Dennison (Yeovil District Hospital NHS Foundation Trust), Rowland Hackett (patient representative), Anne Bennett (Friends of Yeovil Hospital Charity), Emad Salib (Aid Medical), Liz Hawes, Ann Holmes, Sue Robertson, Gail Morrison, Joe Shoebridge, Sam Stefan (Portsmouth NHS Hospitals Trust), Daniel Jennings, Susan Sargent, Matt Dunstan, Claudia Forster (Royal Surrey County Hospital), Nicki Palmer, Leigh Davies, Matthew Williams, James Horwood, Buddug Rees (University Hospital of Wales), Richard Atkinson, Paul Lewis, Fred Dale, Lewis Thorpe, James Sturgess (Karl Storz Endoscopy UK Ltd).
Funding
European Association of Endoscopic Surgeons research grant. 3D laparoscopic systems were provided by Karl Storz Endoscopy (UK) Ltd as a free research loan. Karl Storz had no input to the design, set-up, running, data collection, analysis or preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
N. J. Curtis, J. A. Conti, R. Dalton, T. A. Rockall, A. S. Allison, J. B. Ockrim, I. C. Jourdan, J. Torkington, S. Phillips, J. Allison, G. B. Hanna, and N. K. Francis confirm they hold no conflict of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Curtis, N.J., Conti, J.A., Dalton, R. et al. 2D versus 3D laparoscopic total mesorectal excision: a developmental multicentre randomised controlled trial. Surg Endosc 33, 3370–3383 (2019). https://doi.org/10.1007/s00464-018-06630-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-06630-9