Abstract
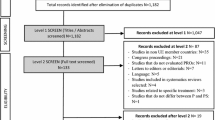
In the course of the chronic skin disease psoriasis, where a variety of treatment interventions is available, a strong growth of health economic studies comparing treatment costs and benefits can be noticed. The objective was to identify health economic evaluations of psoriasis treatments that have been published to date. Of particular interest were the mostly used analysis and outcome parameters, the compared treatments, and the question, if available health economic studies may be used to perform a meta-analysis of qualitative findings. A systematic literature search using PubMed Medline, Ovid Medline, and Cochrane Library was performed for articles, published and available until mid of January 2016. Among the key words were the terms “psoriasis” and “cost-effectiveness”. The search resulted in 318 articles without duplicates. Thereof 60 health economic analyses in psoriasis management were identified. Most of these are cost-effectiveness evaluations (45). The clinical parameter PASI (Psoriasis Area Severity Index) is the most often used cost-effectiveness outcome (33) followed by the Dermatology Life Quality Index (DLQI) (6). In case of cost-utility analyses, QALYs (quality-adjusted life-years) were mostly generated with the help of EuroQol five dimensions questionnaire (EQ-5D) (12), which was partly based on PASI and DLQI values. The majority of health economic studies is focusing on the direct medical and non-medical costs without consideration of productivity losses. Almost 70 % of 60 publications were conducted in Europe. Overall, most considered systemic treatments were the biological agents etanercept (36), adalimumab (27), and infliximab (26) followed by ustekinumab (17) and phototherapy (incl. UV-B, PUVA/psoralen combined with UV-A) (14). Comparisons including only topical treatments mostly focused on vitamin D treatment (14), corticosteroids (13), and coal tar products (6) followed by dithranol (5) and tazarotene (4). Given the setting, compared treatments, and study conditions, different results can be found for medical decision-making. Thereby, it can be noted that there are no standards on methods and outcomes measures available. This leads to a very limited comparability of health economic studies and presents no comfortable basis to examine a meta-analysis of health economic results. The presented systematic review shows the need for nationwide data and interpretation.




Similar content being viewed by others
References
Affleck AG, Bottomley JM, Auland M et al (2011) Cost effectiveness of the two-compound formulation calcipotriol and betamethasone dipropionate gel in the treatment of scalp psoriasis in Scotland. Curr Med Res Opin 27(1):269–284
Aggarwal K, Khandpur S, Khanna N et al (2013) Pandav CS Comparison of clinical and cost-effectiveness of psoralen + ultraviolet A versus psoralen + sunlight in the treatment of chronic plaque psoriasis in a developing economy. Int J Dermatol 52(4):478–485
Ahn CS, Gustafson CJ, Sandoval LF et al (2013) Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol 14(4):315–326
Alora-Palli MB (2010) A cost-effectiveness comparison of liquor carbonis distillate solution and calcipotriol cream in the treatment of moderate chronic plaque psoriasis. Arch Dermatol 146:918
Anis AH, Bansback N, Sizto S et al (2011) Economic evaluation of biologic therapies for the treatment of moderate to severe psoriasis in the United States. J Dermatol Treat 22(2):65–74
Ashcroft DM, Li Wan Po A, Williams HC, Griffiths CE (2000) Cost-effectiveness analysis of topical calcipotriol versus short-contact dithranol. In the treatment of mild to moderate plaque psoriasis. Pharmacoeconomics 18(5):469–476
Augustin M, Peeter P, Radtke M, Moehling U, Lapp C (2007) Cost-effectiveness model of topical treatment of mild to moderate psoriasis vulgaris in Germany. A comparison of calcipotriol/betamethasone (Daivobet/Dovobet/Taclonex) once daily and a morning/evening non-fix combination of calcipotriol and betamethasone. Dermatology (Basel, Switzerland) 215(3):219–228
Augustin M, Radtke M, van Engen A et al (2009) Pharmacoeconomic model of topical treatment options of mild to moderate psoriasis vulgaris in Germany. J Dtsch Dermatol Ges 7(4):329–338
Augustin M, Radtke MA (2010) Gesundheitsökonomie und Versorgung der Psoriasis. UNI-MED, Bremen
Bergstrom KG, Arambula K, Kimball AB (2003) Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05 % compared with a combined program of clobetasol cream 0.05 % and solution 0.05 % for the treatment of psoriasis. Cutis 72(5):407–411
Bottomley JM, Auland ME, Morais J, Boyd G, Douglas WS (2007) Cost-effectiveness of the two-compound formulation calcipotriol and betamethasone dipropionate compared with commonly used topical treatments in the management of moderately severe plaque psoriasis in Scotland. Curr Med Res Opin 23(8):1887–1901
Chen S, Shaheen A, Garber A (1998) Cost-effectiveness and cost-benefit analysis of using methotrexate vs Goeckerman therapy for psoriasis. A pilot study. Arch Dermatol 134(12):1602–1608
Chern E, Yau D, Ho JC et al (2011) Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol 91(4):447–451
Chi C-C, Wang S-H (2014) Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int 2014:862851. doi:10.1155/2014/862851
Colombo G, Bruno M, Girolomoni G, Vena G (2012) Calcipotriol and betamethasone dipropionate in the treatment of mild-to-moderate psoriasis: a cost-effectiveness analysis of the ointment versus gel formulation. Clinicoecon Outcomes Res 4:261-268
Colombo GL, Di Matteo S, Peris K et al (2009) A cost-utility analysis of etanercept for the treatment of moderate-to-severe psoriasis in Italy. Clinicoecon Outcomes Res 1:53–59
Currie CJ, Conway P (2007) PSK11 evaluation of the association between EQ5D utility and dermatology life quality index (DLQI) score in patients with psoriasis. SKIN - Patient Reported Outcomes 10(6):A470–A471
de Portu S, Del Giglio M, Altomare G et al (2010) Cost-effectiveness analysis of TNF-alpha blockers for the treatment of chronic plaque psoriasis in the perspective of the Italian health-care system. Dermatol Ther 23(Suppl 1):S7–S13
Devaux S, Castela A, Archier E et al (2012) Topical vitamin D analogues alone or in association with topical steroids for psoriasis: a systematic review. J Eur Acad Dermatol Venereol: JEADV 26 Suppl 3:52–60
Drummond MF (2014) Methods for the economic evaluation of health care programmes, 3rd edn. Oxford University Press, New York
D’Souza LS, Payette MJ (2015) Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol 72(4):589–598
Ellis CN, Reiter KL, Bandekar RR, Fendrick AM (2002) Cost-effectiveness comparison of therapy for psoriasis with a methotrexate-based regimen versus a rotation regimen of modified cyclosporine and methotrexate. J Am Acad Dermatol 46(2):242–250
Feldman SR, Garton R, Averett W, Balkrishnan R, Vallee J (2003) Strategy to manage the treatment of severe psoriasis: considerations of efficacy, safety and cost. Expert Opin Pharmacother 4(9):1525–1533
Feldman SR, Pearce DJ (2004) Management and costs of severe psoriasis: the role of new biologics. Expert Rev Pharmacoecon Outcomes Res 4(5):573–579
Ferrándiz C, García A, Blasco A, Lázaro P (2012) Cost-efficacy of adalimumab, etanercept, infliximab and ustekinumab for moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol 26:768–777
Freeman K, Marum M, Bottomley JM et al (2011) A psoriasis-specific model to support decision making in practice—UK experience. Curr Med Res Opin 27(1):205–223
Fowler JF, Duh MS, Rovba L et al (2008) The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermartol 59(5):772–780
Greiner RA, Braathen LR (2009) Cost-effectiveness of biologics for moderate-to-severe psoriasis from the perspective of the Swiss healthcare system. Eur J Dermatol 19(5):494–499
Hakkaart-van Roijen L, Verboom P, Redekop WK, Touw KR, Rutten FF (2001) The cost effectiveness of tapered versus abrupt discontinuation of oral cyclosporin microemulsion for the treatment of psoriasis. Pharmacoeconomics 19(5 Pt 2):599–608
Hankin CS, Bhatia ND, Goldenberg G et al (2010) A comparison of the clinical effectiveness and cost-effectiveness of treatments for moderate to severe psoriasis. Drug Benefit Trends 22(1):17–27
Hankin CS, Feldman SR, Szczotka A et al (2005) A cost comparison of treatments of moderate to severe psoriasis. Drug Benefit Trends 17(5):200–214
Hartman M, Prins M, Swinkels OQJ et al (2002) Cost-effectiveness analysis of a psoriasis care instruction programme with dithranol compared with UVB phototherapy and inpatient dithranol treatment. Br J Dermatol 147(3):538–544
Heinen-Kammerer T, Daniel D, Stratmann L, Rychlik R, Boehncke WH (2007) Cost-effectiveness of psoriasis therapy with etanercept in Germany. Journal der Deutschen Dermatologischen Gesellschaft = J Ger Soc Dermatol: JDDG 5(9):762–768
Icks A, Chernyak N, Bestehorn K et al (2010) Methoden der gesundheitsökonomischen Evaluation in der Versorgungsforschung. Gesundheitswesen 72(12):917–933
Igarashi A, Kuwabara H, Fahrbach K, Schenkel B (2013) Cost-efficacy comparison of biological therapies for patients with moderate to severe psoriasis in Japan. J Dermatol Treat 24(5):351–355
Institute for Quality and Efficiency in Health Care (IQWiG) (2009) Working paper modelling version 1.0. Available via Dialog. https://www.iqwig.de/download/Working_Paper_Modelling.pdf. Accessed 27 Sept 2013
Institute for Quality and Efficiency in Health Care (IQWiG) (2009) General methods for the assessment of the relation of benefits to costs: version 1.0. IQWIG, Köln
Knight C, Mauskopf J, Ekelund M, Singh A, Yang S, Boggs R (2012) Cost-effectiveness of treatment with etanercept for psoriasis in Sweden. Eur J Health Econ 13(2):145–156
Koek MB, Sigurdsson V, van Weelden H et al (2010) Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ 340:1490
Liu Y, Wu EQ, Bensimon AG et al (2012) Cost per responder associated with biologic therapies for Crohn’s disease, psoriasis, and rheumatoid arthritis. Adv Ther 29(7):620–634
Lloyd A, Reeves P, Conway P, Reynolds A, Baxter G (2009) Economic evaluation of etanercept in the management of chronic plaque psoriasis. Br J Dermatol 160(2):380–386
Marchetti A, LaPensee K, An P (1998) A pharmacoeconomic analysis of topical therapies for patients with mild-to-moderate stable plaque psoriasis: a US study. Clin Ther 20(4):851–869
Marchetti A, Feldman SR, Kimball AB et al (2005) Treatments for mild-to-moderate recalcitrant plaque psoriasis: expected clinical and economic outcomes for first-line and second-line care. Dermatol Online J 11(1):1
Martin S, Feldman SR, Augustin M, Szapary P, Schenkel B (2011) Cost per responder analysis of ustekinumab and etanercept for moderate to severe plaque psoriasis. J Dermatol Treat 22(3):138–143
Nast A, Boehncke WH, Mrowietz U et al (2012) S3—guidelines on the treatment of psoriasis vulgaris (English version). Update. Journal der Deutschen Dermatologischen Gesellschaft = J Ger Soc Dermatol: JDDG 10 Suppl 2:S1–S95
National Institute for Health and Care Excellence (NICE) (2014) Measuring effectiveness and cost effectiveness: the QALY. Available via Dialog. https://www.nice.org.uk/proxy/?sourceurl=http://www.nice.org.uk/newsroom/features/measuringeffectivenessandcosteffectivenesstheqaly.jsp. Accessed 22 May 2014
National Institute for Health and Care Excellence (NICE) (2013) Guide to the methods of technology appraisal 2013. Available via Dialog. https://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf. Accessed 24 April 2014
Nelson AA, Pearce DJ, Fleischer AB, Balkrishnan R, Feldman SR (2006) New treatments for psoriasis: which biologic is best? [Review] [42 refs]. J Dermatol Treat 17(2):96–107
Nelson AA, Pearce DJ, Fleischer AB, Balkrishnan R, Feldman SR (2008) Cost-effectiveness of biologic treatments for psoriasis based on subjective and objective efficacy measures assessed over a 12-week treatment period. J Am Acad Dermatol 58(1):125–135
Pan F, Brazier NC, Shear NH, Jivraj F, Schenkel B, Brown R (2011) Cost utility analysis based on a head-to-head Phase 3 trial comparing ustekinumab and etanercept in patients with moderate-to-severe plaque psoriasis: a Canadian perspective. Value Health 4(5):652–656
Papp K, Poulin Y, Barber K et al (2012) Cost-effectiveness evaluation of clobetasol propionate shampoo (CPS) maintenance in patients with moderate scalp psoriasis: a Pan-European analysis. J Eur Acad Dermatol Venereol 26(11):1407–1414
Pearce DJ, Nelson AA, Fleischer AB, Balkrishnan R, Feldman SR (2006) The cost-effectiveness and cost of treatment failures associated with systemic psoriasis therapies. J Dermatol Treat 17(1):29–37
Peeters P, Ortonne JP, Sitbon R, Guignard E (2005) Cost-effectiveness of once-daily treatment with calcipotriol/betamethasone dipropionate followed by calcipotriol alone compared with tacalcitol in the treatment of Psoriasis vulgaris. Dermatology (Basel, Switzerland) 211(2):139–145
Polistena B, Calzavara-Pinton P, Altomare G et al (2015) The impact of biologic therapy in chronic plaque psoriasis from a societal perspective: an analysis based on Italian actual clinical practice. J Eur Acad Dermatol Venereol: JEADV 29(12):2411–2416
Puig L, López-Ferrer A, Vilarrasa E (2014) Incremental cost-effectiveness ratio analysis of biologic treatments for psoriasis at clinically significant evaluation time points. Actas dermo-sifiliográficas 105(10):951–953
Puig L, Lopez-Ferrer A, Vilarrasa E, Garcia I, Fernandez-Del Olmo R (2016) Model for assessing the efficiency of biologic drugs in the treatment of moderate to severe psoriasis for one year in clinical practice in Spain. Actas dermo-sifiliograficas 107(1):34–43
Radtke MA, Augustin M (2014) Biosimilars in psoriasis: what can we expect? Journal der Deutschen Dermatologischen Gesellschaft = J Ger Soc Dermatol: JDDG 12(4):306–312
Radtke MA, Augustin M (2008) Economic considerations in psoriasis management. Clin Dermatol 26:424–431
Riveros BS, Ziegelmann PK, Correr CJ (2014) Cost-effectiveness of biologic agents in the treatment of moderate-to-severe psoriasis. A Brazilian public health service perspective. Value Health Reg Issues 5:65–72
Ruano J, Isla-Tejera B, Jiménez-Puya R (2013) Long-term cost-effectiveness analysis of etanercept and adalimumab for plaque psoriasis not associated with arthritis. Dermatol Ther 3(2):131–142
Sawyer L, Samarasekera EJ, Wonderling D, Smith CH (2013) Topical therapies for the treatment of localized plaque psoriasis in primary care: a cost-effectiveness analysis. Br J Dermatol 168(5):1095–1105
Sawyer LM, Wonderling D, Jackson K, Murphy R, Samarasekera EJ, Smith CH (2015) Biological therapies for the treatment of severe psoriasis in patients with previous exposure to biological therapy: a cost-effectiveness analysis. Pharmacoeconomics 33(2):163–177
Schmitt-Rau K, Rosenbach T, Radtke M, Augustin M (2010) Cost-effectiveness of biological therapy in remission induction of moderate to severe plaque psoriasis. Dermatology 221:236–242
von der Schulenburg JG, Greiner W, Jost F et al (2008) German recommendations on health economic evaluation: third and updated version of the hanover consensus. Value Health 11(4):539–544
Shani J, Harari M, Hristakieva E, Seidl V, Bar-Giyora J (1999) Dead-Sea climatotherapy versus other modalities of treatment for psoriasis: comparative cost-effectiveness. Int J Dermatol 38(4):252–262
Sizto S, Bansback N, Feldman SR, Willian MK, Anis AH (2009) Economic evaluation of systemic therapies for moderate to severe psoriasis. Br J Dermatol 160(6):1264–1272
Spandonaro F, Ayala F, Berardesca E et al (2014) The cost effectiveness of biologic therapy for the treatment of chronic plaque psoriasis in real practice settings in Italy. BioDrugs 28(3):285–295
Staidle JP, Dabade TS, Feldman SR (2011) A pharmacoeconomic analysis of severe psoriasis therapy: a review of treatment choices and cost efficiency. Expert Opin Pharmacother 12(13):2041–2054
Stern RS (1988) The benefits, costs and risks of topical tar preparations in the treatment of psoriasis: considerations of cost effectiveness. Ann Acad Med Singap 17(4):473–476
Terranova L, Mattozzi C, Richetta AG et al (2014) Costs of therapy with biologics in the treatment of moderate to severe plaque psoriasis in the context of the Italian health-care system. G Ital Dermatol Venereol 149(1):131–143
Vañó-Galván S, Gárate MT, Fleta-Asín B (2012) Analysis of the cost effectiveness of home-based phototherapy with narrow-band UV-B radiation compared with biological drugs for the treatment of moderate to severe psoriasis. Actas Dermosifiliogr 103(2):127–137
Villacorta R, Hay JW, Messali A (2013) Cost effectiveness of moderate to severe psoriasis therapy with etanercept and ustekinumab in the United States. Pharmacoeconomics 31(9):823–839
Wang SH, Chi CC, Hu S (2014) Cost-efficacy of biologic therapies for moderate to severe psoriasis from the perspective of the Taiwanese healthcare system. Int J Dermatol 53(9):1151–1156
Woolacott N, Hawkins N, Mason A et al (2006) Etanercept and efalizumab for the treatment of psoriasis: a systematic review. [Review] [77 refs]. Health Technol Assess (Winch, Engl) 10(46):1–233
Related articles recently published in Archives of Dermatological Research (selected by the journal’s editorial staff)
Blome C, Gosau R, Radtke MA, Reich K, Rustenbach SJ, Spehr C, Thaci D, Augustin M (2016) Patient-relevant treatment goals in psoriasis. Arch Dermatol Res 308:69–78
Hawro T, Maurer M, Hawro M, Kaszuba A, Cierpialkowska L, Krolikowska M, Zalewska A (2014) In psoriasis, levels of hope and quality of life are linked. Arch Dermatol Res 306:661–666
Kuster D, Nast A, Gerdes S, Weberschock T, Wozel G, Gutknecht M, Schmitt J (2016) Cost-effectiveness of systemic treatments for moderate-to-severe psoriasis in the German health care setting. Arch Dermatol Res 308:249–261
Radtke MA, Reich K, Spehr C, Augustin M (2015) Treatment goals in psoriasis routine care. Arch Dermatol Res 307:445–449
Reich K, Mrowietz U, Radtke MA, Thaci D, Rustenbach SJ, Spehr C, Augustin M (2015) Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry PsoBest. Arch Dermatol Res 307:875–883
Schmitt J, Kuster D (2015) Correlation between Dermatology Life Quality Index (DLQI) scores and Work Limitations Questionnaire (WLQ) allows the calculation of percent work productivity loss in patients with psoriasis. Arch Dermatol Res 307:451–453
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Augustin M has served as consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis including Abbott, Almirall, Amgen, Biogen, Celgene, Centocor, Janssen-Cilag, Leo, Medac, MSD (formerly Essex, Schering-Plough), Novartis, Pfizer (formerly Wyeth). Gutknecht M and Krensel M don’t have any conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Gutknecht, M., Krensel, M. & Augustin, M. Health economic analyses of psoriasis management: a systematic literature search. Arch Dermatol Res 308, 601–616 (2016). https://doi.org/10.1007/s00403-016-1673-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-016-1673-4