-
PDF
- Split View
-
Views
-
Cite
Cite
G Huang, X Chen, W Y Lau, F Shen, R-Y Wang, S-X Yuan, W-X Geng, W-P Zhou, Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas, British Journal of Surgery, Volume 101, Issue 8, July 2014, Pages 1006–1015, https://doi.org/10.1002/bjs.9539
Close - Share Icon Share
Abstract
Health-related quality of life (HRQL) is an important outcome measure in studies of cancer therapy. This study aimed to investigate HRQL and survival in patients with small hepatocellular carcinoma (HCC) treated with either surgical resection or percutaneous radiofrequency ablation (RFA).
Between January 2006 and June 2009, patients with newly diagnosed solitary, small (3 cm or less) HCC were invited to participate in this non-randomized prospective parallel cohort study. The Functional Assessment of Cancer Therapy – Hepatobiliary (FACT-Hep) instrument was used for assessing HRQL. HRQL and survival were compared between the two treatment groups.
A total of 389 patients were enrolled. Questionnaires were completed fully by 99·7 per cent of invited participants (388 of 389) at baseline, 98·7 per cent (383 of 388) at 3 months, 99·0 per cent (379 of 383) at 6 months, 98·4 per cent (365 of 371) at 1 year, 96·6 per cent (336 of 348) at 2 years and 95·1 per cent (289 of 304) at 3 years. There were no significant differences in disease-free and overall survival between the two groups. Patients treated with percutaneous RFA had significantly better HRQL total scores after 3, 6, 12, 24 and 36 months than those who had surgical resection (P < 0·001, P < 0·001, P = 0·001, P = 0·003 and P = 0·025 respectively). On multivariable analysis, the presence of concomitant disease, cirrhosis and surgical resection were significant risk factors associated with a worse HRQL score after treatment.
Percutaneous RFA produced better post-treatment HRQL than surgical resection for patients with solitary small (no more than 3 cm) HCC.
Introduction
Hepatocellular carcinoma (HCC) is the fifth commonest cancer and the third most common cause of cancer-related death in the world1. Surgical resection and local ablation (percutaneous radiofrequency ablation, RFA) are now considered as curative treatments for patients with early-stage HCC2. Survival after percutaneous RFA for early-stage HCC is now comparable to that after surgical resection3–6. However, there is a lack of information on quality of life after percutaneous RFA compared with surgical resection for early-stage HCC. Health-related quality-of-life (HRQL) assessment measures the quality of various aspects of a patient's life, commonly including physical, psychological and social domains. HRQL assessment has been acknowledged as an important endpoint in clinical cancer trials and clinical practice, along with the traditional endpoints of disease-free and overall survival7,8. Impairment in HRQL has been reported in patients with chronic liver disease and HCC9–12. Surgical resection for HCC has been shown to have a major influence on patients' HRQL13–15. This study aimed to compare HRQL and long-term survival after surgical resection and percutaneous RFA for solitary small (3 cm or less) HCC.
Methods
This was a prospective non-randomized study of patients with hepatitis B-related small HCC who underwent therapy with curative intent at the Liver Unit of the Eastern Hepatobiliary Surgery Hospital between January 2006 and June 2009. Patients with a solitary HCC no more than 3 cm in diameter were considered for inclusion. The diagnosis of HCC was based on the diagnostic criteria for HCC used by the European Association for the Study of the Liver16.
Inclusion criteria were: age 18–75 years; solitary HCC with a diameter of 3 cm or less; Child–Pugh grade A; no extrahepatic metastases; no invasion of the portal vein, hepatic vein or their branches; hepatitis B surface antigen (HBsAg) positivity; no coagulopathy, with platelet count exceeding 50 × 109/l and prothrombin time of below 5 s; no history of malignancy; no cognitive impairment or problems with language communication; and ability to self-complete questionnaires.
The decision to treat tumours with either percutaneous RFA or surgical resection was made by the patients themselves after full communication with the treating clinicians. All patients meeting the above inclusion criteria were approached by their clinicians to determine whether they were interested to learn more about the purpose, risks and benefits of the study. If the patient agreed, a clinical psychologist explained the study in detail and obtained informed consent for participation. On receipt of written informed consent, patients were asked to complete a series of questionnaires. This study was approved by the ethics committee of the Eastern Hepatobiliary Surgery Hospital.
Preoperative investigations
All patients underwent chest X-ray, ultrasonography and contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen. Further investigations were performed only if there was suspicion of extrahepatic metastases.
Routine laboratory blood tests were carried out including measurement of HBsAg, hepatitis C virus (HCV) antibody, hepatitis B e antigen (HBeAg) and its antibody, hepatitis B virus (HBV) DNA level, serum α-fetoprotein (AFP), serum albumin, serum total bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT) and prothrombin time.
Surgical procedure
Surgical resection was carried out under general anaesthesia through a right subcostal incision. The abdominal cavity was searched carefully for extent of local disease and extrahepatic metastases. Intraoperative ultrasonography was used to assess the number and size of the lesions, and the relationship between the tumour and vascular structures. Pringle's manoeuvre was applied to occlude the blood inflow of the liver with cycles of 15 min clamping and 5 min unclamping. Hepatic parenchymal transection was carried out using the clamp crushing method.
Percutaneous radiofrequency ablation
Tumour size and ablation zone were evaluated by contrast-enhanced CT and/or contrast-enhanced MRI before treatment. Percutaneous RFA was carried out under ultrasound guidance using a Celonlab power control unit (Olympus, Tokyo, Japan) with a Levine needle, or a Cool-tip™ RFA system (Valleylab, Boulder, Colorado, USA) using local anaesthetic or intravenous sedation. Treatment efficacy was assessed by contrast-enhanced CT and/or MRI after 1 month showing absence of pathological enhancement within, or at the edge of, the treated HCC. If there was residual tumour tissue, additional treatment with percutaneous RFA was given.
Follow-up
All patients were followed up for 36 months. In-hospital mortality was defined as death within the same hospital admission after treatment. Any treatment-related complications or adverse events were recorded. Postoperative liver failure was defined using the ‘50–50’ criteria on day 5 after surgery17. Patients were followed up at monthly intervals during the first year, and then once every 3 months. At each follow-up visit, adverse events were documented and blood was taken for blood counts, coagulation profile, renal and liver function, and AFP and HBV DNA level. Ultrasound examination was done monthly, and chest X-ray and CT or MRI once every 3 months.
HRQL was assessed at baseline, and at 3, 6, 12, 24 and 36 months after treatment. The primary endpoint of the study was change in HRQL. Secondary endpoints were disease-free and overall survival rates. Postoperative morbidity, mortality and length of hospital stay were also compared.
Health-related quality-of-life survey
Patients were asked to complete a Chinese translation of the Functional Assessment of Cancer Therapy – Hepatobiliary (FACT-Hep) questionnaire before treatment and at the scheduled follow-up appointments. The FACT-Hep is a validated instrument and is used widely for assessing HRQL in patients with hepatobiliary cancers18–20. It is a 45-item self-reported instrument that consists of the 27-item FACT – General (FACT-G), which assesses generic HRQL concerns using four subscales, and the 18-item hepatobiliary cancer subscale (HepCS), which assesses specific symptoms of hepatobiliary cancer and side-effects of treatment. The FACT-Hep measures HRQL in five domains: physical well-being, functional well-being and social/family well-being each contain seven items with a subscale score of 0–28 points; emotional well-being contains six items with a subscale score of 0–24 points; and the HepCS contains 18 items with a subscale score of 0–72 points. The present study also compared the trial outcome index (TOI), which comprises a summation of the physical well-being, functional well-being and HepCS subscales, and is a sensitive indicator of clinical outcome in other disease types21. The patients circled numbers on a scale from 0 (not at all) to 4 (very much) to indicate their reaction to each statement. The FACT-G and HepCS scores were summed to obtain the FACT-Hep total score, which ranged from 0 to 180. At each time point, the scores on the FACT physical, social/family, emotional and functional well-being subscales, HepCS, TOI and FACT-Hep total scores were compared between the two groups. Higher scores on all subscales of the FACT-Hep indicated better HRQL and fewer symptoms.
The FACT-Hep shows a high internal consistency at initial assessment (Cronbach's α range 0·72–0·94) and retesting (Cronbach's α range 0·81–0·94)18. Measurement stability is also high for all aggregated scales (test–retest correlation range 0·84–0·91; interclass correlation coefficient range 0·82–0·90)18. The FACT-Hep can be used independently as a brief measure of disease-related symptoms and functioning in assessing HRQL of patients with HCC18. Studies using the Chinese version of the FACT-G and FACT-Hep showed better reliability and validity18,22–23.
All HRQL data were collected by a trained research assistant. To minimize bias, patients were informed beforehand that responses to questionnaires would not be revealed to the attending surgeons. The assistant who calculated the HRQL scores was blinded to the patients' clinical and sociodemographic information. Differences between groups were compared not only for each subscale of FACT-Hep, but also for the FACT-Hep total score. Considering that the FACT-Hep total score should be more comprehensive and effective in evaluating HRQL, this total score was chosen as the primary endpoint. The completeness and quality of individual patient data were evaluated by a research assistant when the patient completed all the questionnaire forms at the scheduled follow-up appointments, and this did not add an extra burden to the patient. Patients who missed an appointment were contacted by telephone and sent follow-up questionnaires by mail. For patients who survived for less than 3 years after treatment, HRQL assessment was performed until last follow-up.
Statistical analysis
Clinicopathological data, laboratory blood tests, FACT-Hep scores and recurrence status after treatment were entered prospectively into a computerized database. A total of 0·3 per cent of the data were missing. The missing data were managed by the mean imputation method. Continuous data are expressed as mean(s.d.) or median (range). These were compared using Student's t test or the non-parametric Mann–Whitney U test. Categorical variables were tested by means of χ2 or Fisher's exact test. Repeated-measures ANOVA was used to compare serial measurements of HRQL scores from baseline to 3-year follow-up. Cumulative recurrence rates were calculated by the Kaplan–Meier method and differences compared by log rank test. Univariable and multivariable logistic regression analyses were performed. Variables (including sociodemographic, disease and treatment factors) that significantly influenced post-treatment HRQL in univariable analysis (P < 0·100) were included in the multivariable analysis. Stepwise logistic regression analyses were carried out to identify variables associated with the HRQL score. Statistical significance was defined as P < 0·050. Statistical analyses were done using SPSS® version 18.0 for Windows® (IBM, Armonk, New York, USA).
Results
Among 4337 patients with a diagnosis of HCC who underwent treatment with curative intent during the study period, 414 met the inclusion criteria. Twenty-five patients (6·0 per cent) were excluded: four (1·0 per cent) were seropositive for both HBsAg and HCV antibody; eight (1·9 per cent) were found not to have HCC on histopathology of resected specimens; three (0·7 per cent) had tumour recurrence or residual tumour after percutaneous RFA, and chose surgical resection; and ten (2·4 per cent) refused to participate.
The remaining 389 patients received the FACT-Hep questionnaires. Questionnaires were completed by 99·7 per cent of invited participants (388 of 389) at baseline, 98·7 per cent (383 of 388) at 3 months, 99·0 per cent (379 of 383) at 6 months, 98·4 per cent (365 of 371) at 1 year, 96·6 per cent (336 of 348) at 2 years and 95·1 per cent (289 of 304) at 3 years. Reminders (mainly via telephone) prompted responses effectively. There was no significant difference in response rates between the two groups. Forty-three patients did not complete the HRQL survey fully at all six time points, and were removed from the study (21 were unable to continue the study during follow-up, 17 were lost to follow-up and 5 did not turn up to complete all questionnaires).
The remaining 346 patients were finally included in this study (Table 1). Some 225 patients underwent surgical resection and 121 percutaneous RFA. In the surgical resection group, the numbers of patients who had R0 and R1 resection were 220 (97·8 per cent) and five (2·2 per cent) respectively. In the RFA group, the mean number of percutaneous RFA treatments was 1·1 (range 1–3). One hundred and sixteen patients (95·9 per cent) showed complete necrosis on the first postoperative CT or MRI scan. Five patients received a second or third percutaneous RFA or percutaneous ethanol injection because of incomplete necrosis. Blood transfusion rates were higher in the surgical resection group. Duration of the procedure and length of hospital stay were significantly longer after surgical resection than percutaneous RFA (Table 1). Otherwise, there were no significant differences between the two groups.
Comparison of demographic and clinical data between percutaneous radiofrequency ablation and surgical resection groups
| . | RFA (n = 121) . | Resection (n = 225) . | P¶ . |
|---|---|---|---|
| Age (years)* | 51·2(11·3) | 50·4(10·0) | 0·483# |
| Sex ratio (M : F) | 106 : 15 | 199 : 26 | 0·817 |
| Concomitant diseases‡ | 28 (23·1) | 40 (17·8) | 0·231 |
| Education | 0·819 | ||
| Elementary school | 16 (13·2) | 35 (15·6) | |
| Middle school or equivalent | 76 (62·8) | 135 (60·0) | |
| University or college | 29 (24·0) | 55 (24·4) | |
| Employment status | 0·403 | ||
| Unemployed | 8 (6·6) | 17 (7·6) | |
| Working (full or part time) | 86 (71·1) | 171 (76·0) | |
| Retired | 27 (22·3) | 37 (16·4) | |
| Marital status | 0·931 | ||
| Unmarried | 2 (1·7) | 2 (0·9) | |
| Married | 110 (90·9) | 206 (91·6) | |
| Divorced or separated | 6 (5·0) | 12 (5·3) | |
| Widowed | 3 (2·5) | 5 (2·2) | |
| Personal monthly income (RMB) | 0·866 | ||
| 0–1999 (€0–234§) | 5 (4·1) | 7 (3·1) | |
| 2000–4999 (€235–585) | 54 (44·6) | 110 (48·9) | |
| 5000–9999 (€586–1170) | 40 (33·1) | 71 (31·6) | |
| ≥ 10 000 (≥ €1171) | 22 (18·2) | 37 (16·4) | |
| HBeAg positivity | 29 (24·0) | 57 (25·3) | 0·779 |
| Preoperative HBV DNA (units /ml) | 0·524 | ||
| < 200 | 73 (60·3) | 135 (60·0) | |
| 200–20 000 | 29 (24·0) | 63 (28·0) | |
| > 20 000 | 19 (15·7) | 27 (12·0) | |
| AFP (ng/ml)† | 221·3 (1·6–1210·0) | 254·5 (2·2–1210·0) | 0·768** |
| Preoperative laboratory tests* | |||
| Haemoglobin (g/l) | 144·4(18·1) | 143·3(18·1) | 0·609# |
| Platelet count (×109/l) | 140·8(62·0) | 149·1(67·4) | 0·266# |
| Prothrombin time (s) | 12·2(1·0) | 12·1(1·1) | 0·194# |
| Bilirubin (µmol/l) | 16·0(3·2) | 15·5(2·8) | 0·143# |
| Albumin (g/l) | 40·2(3·8) | 40·5(3·9) | 0·538# |
| ALT(units/l) | 40·0(13·8) | 39·1(17·3) | 0·619# |
| Creatinine (µmol/l) | 72·7(13·3) | 72·1(13·5) | 0·695# |
| Cirrhosis | 96 (79·3) | 172 (76·4) | 0·539 |
| Tumour diameter (cm)* | 2·5(0·6) | 2·4(0·6) | 0·489 |
| Tumour location (liver segment) | 0·908 | ||
| I | 2 (1·7) | 4 (1·8) | |
| II and III | 13 (10·7) | 31 (13·8) | |
| IV | 14 (11·6) | 22 (9·8) | |
| V and VIII | 43 (35·5) | 74 (32·9) | |
| VI and VII | 49 (40·5) | 94 (41·8) | |
| Duration of operation (min)* | 44·0(14·3) | 166·5(34·6) | < 0·001# |
| Preoperative TAE | 8 (6·6) | 17 (7·6) | 0·746 |
| Antiviral treatment | 25 (20·7) | 41 (18·2) | 0·582 |
| Length of hospital stay (days)* | 7·1(2·7) | 14·5(3·0) | < 0·001# |
| . | RFA (n = 121) . | Resection (n = 225) . | P¶ . |
|---|---|---|---|
| Age (years)* | 51·2(11·3) | 50·4(10·0) | 0·483# |
| Sex ratio (M : F) | 106 : 15 | 199 : 26 | 0·817 |
| Concomitant diseases‡ | 28 (23·1) | 40 (17·8) | 0·231 |
| Education | 0·819 | ||
| Elementary school | 16 (13·2) | 35 (15·6) | |
| Middle school or equivalent | 76 (62·8) | 135 (60·0) | |
| University or college | 29 (24·0) | 55 (24·4) | |
| Employment status | 0·403 | ||
| Unemployed | 8 (6·6) | 17 (7·6) | |
| Working (full or part time) | 86 (71·1) | 171 (76·0) | |
| Retired | 27 (22·3) | 37 (16·4) | |
| Marital status | 0·931 | ||
| Unmarried | 2 (1·7) | 2 (0·9) | |
| Married | 110 (90·9) | 206 (91·6) | |
| Divorced or separated | 6 (5·0) | 12 (5·3) | |
| Widowed | 3 (2·5) | 5 (2·2) | |
| Personal monthly income (RMB) | 0·866 | ||
| 0–1999 (€0–234§) | 5 (4·1) | 7 (3·1) | |
| 2000–4999 (€235–585) | 54 (44·6) | 110 (48·9) | |
| 5000–9999 (€586–1170) | 40 (33·1) | 71 (31·6) | |
| ≥ 10 000 (≥ €1171) | 22 (18·2) | 37 (16·4) | |
| HBeAg positivity | 29 (24·0) | 57 (25·3) | 0·779 |
| Preoperative HBV DNA (units /ml) | 0·524 | ||
| < 200 | 73 (60·3) | 135 (60·0) | |
| 200–20 000 | 29 (24·0) | 63 (28·0) | |
| > 20 000 | 19 (15·7) | 27 (12·0) | |
| AFP (ng/ml)† | 221·3 (1·6–1210·0) | 254·5 (2·2–1210·0) | 0·768** |
| Preoperative laboratory tests* | |||
| Haemoglobin (g/l) | 144·4(18·1) | 143·3(18·1) | 0·609# |
| Platelet count (×109/l) | 140·8(62·0) | 149·1(67·4) | 0·266# |
| Prothrombin time (s) | 12·2(1·0) | 12·1(1·1) | 0·194# |
| Bilirubin (µmol/l) | 16·0(3·2) | 15·5(2·8) | 0·143# |
| Albumin (g/l) | 40·2(3·8) | 40·5(3·9) | 0·538# |
| ALT(units/l) | 40·0(13·8) | 39·1(17·3) | 0·619# |
| Creatinine (µmol/l) | 72·7(13·3) | 72·1(13·5) | 0·695# |
| Cirrhosis | 96 (79·3) | 172 (76·4) | 0·539 |
| Tumour diameter (cm)* | 2·5(0·6) | 2·4(0·6) | 0·489 |
| Tumour location (liver segment) | 0·908 | ||
| I | 2 (1·7) | 4 (1·8) | |
| II and III | 13 (10·7) | 31 (13·8) | |
| IV | 14 (11·6) | 22 (9·8) | |
| V and VIII | 43 (35·5) | 74 (32·9) | |
| VI and VII | 49 (40·5) | 94 (41·8) | |
| Duration of operation (min)* | 44·0(14·3) | 166·5(34·6) | < 0·001# |
| Preoperative TAE | 8 (6·6) | 17 (7·6) | 0·746 |
| Antiviral treatment | 25 (20·7) | 41 (18·2) | 0·582 |
| Length of hospital stay (days)* | 7·1(2·7) | 14·5(3·0) | < 0·001# |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and
median (range).
Hypertension, diabetes mellitus, heart disease and pulmonary disease.
Conversion rate 27 March 2014. RFA, radiofrequency ablation; RMB, renminbi; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; AFP, α-fetoprotein; ALT, alanine aminotransferase; TAE, transarterial embolization.
χ2 test, except
Student's t test and
Mann–Whitney U test.
Comparison of demographic and clinical data between percutaneous radiofrequency ablation and surgical resection groups
| . | RFA (n = 121) . | Resection (n = 225) . | P¶ . |
|---|---|---|---|
| Age (years)* | 51·2(11·3) | 50·4(10·0) | 0·483# |
| Sex ratio (M : F) | 106 : 15 | 199 : 26 | 0·817 |
| Concomitant diseases‡ | 28 (23·1) | 40 (17·8) | 0·231 |
| Education | 0·819 | ||
| Elementary school | 16 (13·2) | 35 (15·6) | |
| Middle school or equivalent | 76 (62·8) | 135 (60·0) | |
| University or college | 29 (24·0) | 55 (24·4) | |
| Employment status | 0·403 | ||
| Unemployed | 8 (6·6) | 17 (7·6) | |
| Working (full or part time) | 86 (71·1) | 171 (76·0) | |
| Retired | 27 (22·3) | 37 (16·4) | |
| Marital status | 0·931 | ||
| Unmarried | 2 (1·7) | 2 (0·9) | |
| Married | 110 (90·9) | 206 (91·6) | |
| Divorced or separated | 6 (5·0) | 12 (5·3) | |
| Widowed | 3 (2·5) | 5 (2·2) | |
| Personal monthly income (RMB) | 0·866 | ||
| 0–1999 (€0–234§) | 5 (4·1) | 7 (3·1) | |
| 2000–4999 (€235–585) | 54 (44·6) | 110 (48·9) | |
| 5000–9999 (€586–1170) | 40 (33·1) | 71 (31·6) | |
| ≥ 10 000 (≥ €1171) | 22 (18·2) | 37 (16·4) | |
| HBeAg positivity | 29 (24·0) | 57 (25·3) | 0·779 |
| Preoperative HBV DNA (units /ml) | 0·524 | ||
| < 200 | 73 (60·3) | 135 (60·0) | |
| 200–20 000 | 29 (24·0) | 63 (28·0) | |
| > 20 000 | 19 (15·7) | 27 (12·0) | |
| AFP (ng/ml)† | 221·3 (1·6–1210·0) | 254·5 (2·2–1210·0) | 0·768** |
| Preoperative laboratory tests* | |||
| Haemoglobin (g/l) | 144·4(18·1) | 143·3(18·1) | 0·609# |
| Platelet count (×109/l) | 140·8(62·0) | 149·1(67·4) | 0·266# |
| Prothrombin time (s) | 12·2(1·0) | 12·1(1·1) | 0·194# |
| Bilirubin (µmol/l) | 16·0(3·2) | 15·5(2·8) | 0·143# |
| Albumin (g/l) | 40·2(3·8) | 40·5(3·9) | 0·538# |
| ALT(units/l) | 40·0(13·8) | 39·1(17·3) | 0·619# |
| Creatinine (µmol/l) | 72·7(13·3) | 72·1(13·5) | 0·695# |
| Cirrhosis | 96 (79·3) | 172 (76·4) | 0·539 |
| Tumour diameter (cm)* | 2·5(0·6) | 2·4(0·6) | 0·489 |
| Tumour location (liver segment) | 0·908 | ||
| I | 2 (1·7) | 4 (1·8) | |
| II and III | 13 (10·7) | 31 (13·8) | |
| IV | 14 (11·6) | 22 (9·8) | |
| V and VIII | 43 (35·5) | 74 (32·9) | |
| VI and VII | 49 (40·5) | 94 (41·8) | |
| Duration of operation (min)* | 44·0(14·3) | 166·5(34·6) | < 0·001# |
| Preoperative TAE | 8 (6·6) | 17 (7·6) | 0·746 |
| Antiviral treatment | 25 (20·7) | 41 (18·2) | 0·582 |
| Length of hospital stay (days)* | 7·1(2·7) | 14·5(3·0) | < 0·001# |
| . | RFA (n = 121) . | Resection (n = 225) . | P¶ . |
|---|---|---|---|
| Age (years)* | 51·2(11·3) | 50·4(10·0) | 0·483# |
| Sex ratio (M : F) | 106 : 15 | 199 : 26 | 0·817 |
| Concomitant diseases‡ | 28 (23·1) | 40 (17·8) | 0·231 |
| Education | 0·819 | ||
| Elementary school | 16 (13·2) | 35 (15·6) | |
| Middle school or equivalent | 76 (62·8) | 135 (60·0) | |
| University or college | 29 (24·0) | 55 (24·4) | |
| Employment status | 0·403 | ||
| Unemployed | 8 (6·6) | 17 (7·6) | |
| Working (full or part time) | 86 (71·1) | 171 (76·0) | |
| Retired | 27 (22·3) | 37 (16·4) | |
| Marital status | 0·931 | ||
| Unmarried | 2 (1·7) | 2 (0·9) | |
| Married | 110 (90·9) | 206 (91·6) | |
| Divorced or separated | 6 (5·0) | 12 (5·3) | |
| Widowed | 3 (2·5) | 5 (2·2) | |
| Personal monthly income (RMB) | 0·866 | ||
| 0–1999 (€0–234§) | 5 (4·1) | 7 (3·1) | |
| 2000–4999 (€235–585) | 54 (44·6) | 110 (48·9) | |
| 5000–9999 (€586–1170) | 40 (33·1) | 71 (31·6) | |
| ≥ 10 000 (≥ €1171) | 22 (18·2) | 37 (16·4) | |
| HBeAg positivity | 29 (24·0) | 57 (25·3) | 0·779 |
| Preoperative HBV DNA (units /ml) | 0·524 | ||
| < 200 | 73 (60·3) | 135 (60·0) | |
| 200–20 000 | 29 (24·0) | 63 (28·0) | |
| > 20 000 | 19 (15·7) | 27 (12·0) | |
| AFP (ng/ml)† | 221·3 (1·6–1210·0) | 254·5 (2·2–1210·0) | 0·768** |
| Preoperative laboratory tests* | |||
| Haemoglobin (g/l) | 144·4(18·1) | 143·3(18·1) | 0·609# |
| Platelet count (×109/l) | 140·8(62·0) | 149·1(67·4) | 0·266# |
| Prothrombin time (s) | 12·2(1·0) | 12·1(1·1) | 0·194# |
| Bilirubin (µmol/l) | 16·0(3·2) | 15·5(2·8) | 0·143# |
| Albumin (g/l) | 40·2(3·8) | 40·5(3·9) | 0·538# |
| ALT(units/l) | 40·0(13·8) | 39·1(17·3) | 0·619# |
| Creatinine (µmol/l) | 72·7(13·3) | 72·1(13·5) | 0·695# |
| Cirrhosis | 96 (79·3) | 172 (76·4) | 0·539 |
| Tumour diameter (cm)* | 2·5(0·6) | 2·4(0·6) | 0·489 |
| Tumour location (liver segment) | 0·908 | ||
| I | 2 (1·7) | 4 (1·8) | |
| II and III | 13 (10·7) | 31 (13·8) | |
| IV | 14 (11·6) | 22 (9·8) | |
| V and VIII | 43 (35·5) | 74 (32·9) | |
| VI and VII | 49 (40·5) | 94 (41·8) | |
| Duration of operation (min)* | 44·0(14·3) | 166·5(34·6) | < 0·001# |
| Preoperative TAE | 8 (6·6) | 17 (7·6) | 0·746 |
| Antiviral treatment | 25 (20·7) | 41 (18·2) | 0·582 |
| Length of hospital stay (days)* | 7·1(2·7) | 14·5(3·0) | < 0·001# |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and
median (range).
Hypertension, diabetes mellitus, heart disease and pulmonary disease.
Conversion rate 27 March 2014. RFA, radiofrequency ablation; RMB, renminbi; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; AFP, α-fetoprotein; ALT, alanine aminotransferase; TAE, transarterial embolization.
χ2 test, except
Student's t test and
Mann–Whitney U test.
Complications and post-treatment liver function
There was no hospital death in either group. The Clavien–Dindo score24 was used to grade post-treatment complications. There were significantly more overall complications, grade I and grade III complications in the surgical resection group than in the percutaneous RFA group (Table 2). Serum ALT and albumin levels were significantly worse on postoperative day 5 in the resection group (Table 2).
Comparison of post-treatment complications and liver function after percutaneous radiofrequency ablation or surgical resection
| . | RFA (n = 121) . | Resection (n = 225) . | P† . |
|---|---|---|---|
| Post-treatment laboratory tests* | |||
| Day 5 | |||
| Prothrombin time (s) | 13·3(1·4) | 13·5(1·4) | 0·175‡ |
| Bilirubin (µmol/l) | 21·7(8·7) | 23·1(11·3) | 0·238‡ |
| Albumin (g/l) | 35·0(3·4) | 33·7(3·4) | 0·001‡ |
| ALT (units/l) | 91·6(45·5) | 133·5(105·5) | < 0·001‡ |
| 36 months | |||
| Prothrombin time (s) | 12·3(1·0) | 12·2(1·0) | 0·749‡ |
| Bilirubin (µmol/l) | 16·2(5·0) | 16·7(4·5) | 0·440‡ |
| Albumin (g/l) | 41·2(3·5) | 41·0(3·6) | 0·678‡ |
| ALT (units/l) | 35·9(11·0) | 36·6(14·7) | 0·685‡ |
| Overall complications | 6 (5·0) | 75 (33·3) | < 0·001 |
| Grade I | |||
| Wound infection or dehiscence | 0 (0) | 10 (4·4) | 0·019 |
| Grade II | |||
| Pneumonia | 0 (0) | 3 (1·3) | 0·202 |
| Grade III | 6 (5·0) | 59 (26·2) | < 0·001 |
| Grade IIIa | 5 (4·1) | 53 (23·6) | < 0·001 |
| Delayed gastric emptying | 0 (0) | 9 (4·0) | 0·026 |
| Pleural effusion requiring tapping | 2 (1·7) | 14 (6·2) | 0·054 |
| Biliary leakage | 0 (0) | 10 (4·4) | 0·019 |
| Intra-abdominal abscess | 0 (0) | 5 (2·2) | 0·099 |
| Moderate/severe ascites | 0 (0) | 15 (6·7) | 0·004 |
| Pneumothorax or haemothorax | 2 (1·7) | 0 (0) | 0·053 |
| Liver abscess | 1 (0·8) | 0 (0) | 0·172 |
| Grade IIIb | |||
| Intra-abdominal haemorrhage | 1 (0·8) | 6(2·7) | 0·246 |
| Grade IV | 0 (0) | 3 (1·3) | 0·202 |
| Liver failure | 0 (0) | 2 (0·9) | 0·298 |
| Renal insufficiency | 0 (0) | 1 (0·4) | 0·463 |
| Grade V | 0 (0) | 0 (0) |
| . | RFA (n = 121) . | Resection (n = 225) . | P† . |
|---|---|---|---|
| Post-treatment laboratory tests* | |||
| Day 5 | |||
| Prothrombin time (s) | 13·3(1·4) | 13·5(1·4) | 0·175‡ |
| Bilirubin (µmol/l) | 21·7(8·7) | 23·1(11·3) | 0·238‡ |
| Albumin (g/l) | 35·0(3·4) | 33·7(3·4) | 0·001‡ |
| ALT (units/l) | 91·6(45·5) | 133·5(105·5) | < 0·001‡ |
| 36 months | |||
| Prothrombin time (s) | 12·3(1·0) | 12·2(1·0) | 0·749‡ |
| Bilirubin (µmol/l) | 16·2(5·0) | 16·7(4·5) | 0·440‡ |
| Albumin (g/l) | 41·2(3·5) | 41·0(3·6) | 0·678‡ |
| ALT (units/l) | 35·9(11·0) | 36·6(14·7) | 0·685‡ |
| Overall complications | 6 (5·0) | 75 (33·3) | < 0·001 |
| Grade I | |||
| Wound infection or dehiscence | 0 (0) | 10 (4·4) | 0·019 |
| Grade II | |||
| Pneumonia | 0 (0) | 3 (1·3) | 0·202 |
| Grade III | 6 (5·0) | 59 (26·2) | < 0·001 |
| Grade IIIa | 5 (4·1) | 53 (23·6) | < 0·001 |
| Delayed gastric emptying | 0 (0) | 9 (4·0) | 0·026 |
| Pleural effusion requiring tapping | 2 (1·7) | 14 (6·2) | 0·054 |
| Biliary leakage | 0 (0) | 10 (4·4) | 0·019 |
| Intra-abdominal abscess | 0 (0) | 5 (2·2) | 0·099 |
| Moderate/severe ascites | 0 (0) | 15 (6·7) | 0·004 |
| Pneumothorax or haemothorax | 2 (1·7) | 0 (0) | 0·053 |
| Liver abscess | 1 (0·8) | 0 (0) | 0·172 |
| Grade IIIb | |||
| Intra-abdominal haemorrhage | 1 (0·8) | 6(2·7) | 0·246 |
| Grade IV | 0 (0) | 3 (1·3) | 0·202 |
| Liver failure | 0 (0) | 2 (0·9) | 0·298 |
| Renal insufficiency | 0 (0) | 1 (0·4) | 0·463 |
| Grade V | 0 (0) | 0 (0) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). RFA, radiofrequency ablation; ALT, alanine aminotransferase.
χ2 test, except
Student's t test.
Comparison of post-treatment complications and liver function after percutaneous radiofrequency ablation or surgical resection
| . | RFA (n = 121) . | Resection (n = 225) . | P† . |
|---|---|---|---|
| Post-treatment laboratory tests* | |||
| Day 5 | |||
| Prothrombin time (s) | 13·3(1·4) | 13·5(1·4) | 0·175‡ |
| Bilirubin (µmol/l) | 21·7(8·7) | 23·1(11·3) | 0·238‡ |
| Albumin (g/l) | 35·0(3·4) | 33·7(3·4) | 0·001‡ |
| ALT (units/l) | 91·6(45·5) | 133·5(105·5) | < 0·001‡ |
| 36 months | |||
| Prothrombin time (s) | 12·3(1·0) | 12·2(1·0) | 0·749‡ |
| Bilirubin (µmol/l) | 16·2(5·0) | 16·7(4·5) | 0·440‡ |
| Albumin (g/l) | 41·2(3·5) | 41·0(3·6) | 0·678‡ |
| ALT (units/l) | 35·9(11·0) | 36·6(14·7) | 0·685‡ |
| Overall complications | 6 (5·0) | 75 (33·3) | < 0·001 |
| Grade I | |||
| Wound infection or dehiscence | 0 (0) | 10 (4·4) | 0·019 |
| Grade II | |||
| Pneumonia | 0 (0) | 3 (1·3) | 0·202 |
| Grade III | 6 (5·0) | 59 (26·2) | < 0·001 |
| Grade IIIa | 5 (4·1) | 53 (23·6) | < 0·001 |
| Delayed gastric emptying | 0 (0) | 9 (4·0) | 0·026 |
| Pleural effusion requiring tapping | 2 (1·7) | 14 (6·2) | 0·054 |
| Biliary leakage | 0 (0) | 10 (4·4) | 0·019 |
| Intra-abdominal abscess | 0 (0) | 5 (2·2) | 0·099 |
| Moderate/severe ascites | 0 (0) | 15 (6·7) | 0·004 |
| Pneumothorax or haemothorax | 2 (1·7) | 0 (0) | 0·053 |
| Liver abscess | 1 (0·8) | 0 (0) | 0·172 |
| Grade IIIb | |||
| Intra-abdominal haemorrhage | 1 (0·8) | 6(2·7) | 0·246 |
| Grade IV | 0 (0) | 3 (1·3) | 0·202 |
| Liver failure | 0 (0) | 2 (0·9) | 0·298 |
| Renal insufficiency | 0 (0) | 1 (0·4) | 0·463 |
| Grade V | 0 (0) | 0 (0) |
| . | RFA (n = 121) . | Resection (n = 225) . | P† . |
|---|---|---|---|
| Post-treatment laboratory tests* | |||
| Day 5 | |||
| Prothrombin time (s) | 13·3(1·4) | 13·5(1·4) | 0·175‡ |
| Bilirubin (µmol/l) | 21·7(8·7) | 23·1(11·3) | 0·238‡ |
| Albumin (g/l) | 35·0(3·4) | 33·7(3·4) | 0·001‡ |
| ALT (units/l) | 91·6(45·5) | 133·5(105·5) | < 0·001‡ |
| 36 months | |||
| Prothrombin time (s) | 12·3(1·0) | 12·2(1·0) | 0·749‡ |
| Bilirubin (µmol/l) | 16·2(5·0) | 16·7(4·5) | 0·440‡ |
| Albumin (g/l) | 41·2(3·5) | 41·0(3·6) | 0·678‡ |
| ALT (units/l) | 35·9(11·0) | 36·6(14·7) | 0·685‡ |
| Overall complications | 6 (5·0) | 75 (33·3) | < 0·001 |
| Grade I | |||
| Wound infection or dehiscence | 0 (0) | 10 (4·4) | 0·019 |
| Grade II | |||
| Pneumonia | 0 (0) | 3 (1·3) | 0·202 |
| Grade III | 6 (5·0) | 59 (26·2) | < 0·001 |
| Grade IIIa | 5 (4·1) | 53 (23·6) | < 0·001 |
| Delayed gastric emptying | 0 (0) | 9 (4·0) | 0·026 |
| Pleural effusion requiring tapping | 2 (1·7) | 14 (6·2) | 0·054 |
| Biliary leakage | 0 (0) | 10 (4·4) | 0·019 |
| Intra-abdominal abscess | 0 (0) | 5 (2·2) | 0·099 |
| Moderate/severe ascites | 0 (0) | 15 (6·7) | 0·004 |
| Pneumothorax or haemothorax | 2 (1·7) | 0 (0) | 0·053 |
| Liver abscess | 1 (0·8) | 0 (0) | 0·172 |
| Grade IIIb | |||
| Intra-abdominal haemorrhage | 1 (0·8) | 6(2·7) | 0·246 |
| Grade IV | 0 (0) | 3 (1·3) | 0·202 |
| Liver failure | 0 (0) | 2 (0·9) | 0·298 |
| Renal insufficiency | 0 (0) | 1 (0·4) | 0·463 |
| Grade V | 0 (0) | 0 (0) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). RFA, radiofrequency ablation; ALT, alanine aminotransferase.
χ2 test, except
Student's t test.
Survival
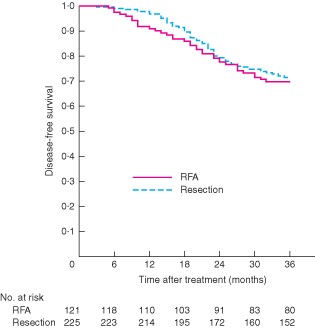
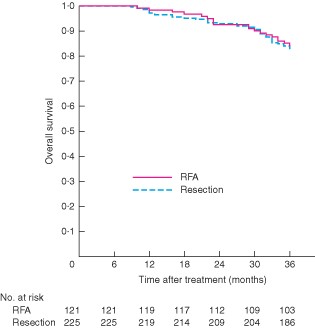
At the time of censoring on 30 June 2012, 100 patients (28·9 per cent) had developed tumour recurrence and 57 (16·5 per cent) had died. Fifty-two patients (91 per cent) died from tumour recurrence and five (9 per cent) from liver failure. There were no significant differences in disease-free and overall survival between the two groups (P = 0·682 and P = 0·570 respectively) (Figs 1 and 2).
Comparison of disease-free survival between percutaneous radiofrequency ablation (RFA) and surgical resection groups. P = 0·682 (log rank test)
Comparison of overall survival between percutaneous radiofrequency ablation (RFA) and surgical resection groups. P = 0·570 (log rank test)
Comparison of health-related quality of life between groups
No patients died before completion of the 3- and 6-month HRQL assessment. Eight of 346 patients died before completion of the 1-year HRQL assessment, 25 (including those who died within 1 year) before completion of the 2-year assessment, and a total of 57 patients before completion of the 3-year assessment.
There were no significant differences in HRQL between the two groups at baseline. However, at 3 months after treatment, with the exception of the social/family well-being scores, all other subscale scores, TOI and the FACT-Hep total scores were significantly lower (worse, with more symptoms and lower functional status) in the surgical resection group (total HRQL scores: P < 0·001, P < 0·001, P = 0·001, P = 0·003 and P = 0·025 after 3, 6, 12, 24 and 36 months) (Table S1, supporting information). At 6, 12, 24 and 36 months after treatment, with the exception of social/family and emotional well-being scores, all other subscale scores and the FACT-Hep total score were significantly lower in the surgical resection group.
Changes in health-related quality of life after treatment
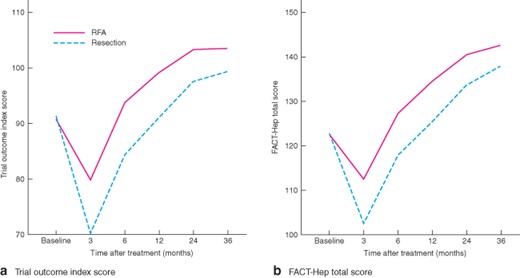
Changes in health-related quality of life after treatment are shown in Fig. 3. Repeated-measures ANOVA showed significant overall differences between treatments over time (from baseline to 36 months) on most of the subscales (physical well-being, functional well-being, HepCS and TOI) of the FACT-Hep (all P < 0·001) (Figs S1–S3, supporting information; Fig. 3a) and total score (P < 0·001) (Fig. 3b), with the exception of social/family and emotional well-being (P = 0·738 and P = 0·069) (Figs S4 and S5, supporting information). HRQL scores were significantly lower in the surgical resection group compared with the percutaneous RFA group in domains such as physical and functional well-being, and disease-specific assessments from 3 to 36 months after treatment (all P < 0·001) (Figs S3–S5, supporting information). The TOI and total score were also significantly lower (both P < 0·001) (Fig. 3). Patients who had surgical resection had a greater decline in physical, functional and disease-specific well-being subscales, TOI and FACT-Hep total scores from baseline to 3 months compared with patients who had percutaneous RFA. After that, there was a continuous and significant improvement in these five scores in both study groups from 3 to 36 months. The peak value of these five subscales in the percutaneous RFA group was higher and appeared earlier than in the surgical group.
Change in Functional Assessment of Cancer Therapy – Hepatobiliary (FACT-Hep) scores from baseline to 36 months after percutaneous radiofrequency ablation (RFA; 121 patients) or surgical resection (225 patients): a trial outcome index scores and b FACT-Hep total scores
Multivariable analysis
Tumour recurrence rates were similar in the two groups. As tumour recurrence may influence HRQL13,20, 246 patients without recurrence were selected for multivariable analysis. A high HRQL score was defined as a post-treatment FACT-Hep total score greater than or equal to the median score of all patients, which was 128. Lower scores indicated worse HRQL.
On multivariable analysis, the presence of concomitant diseases (odds ratio (OR) 2·97, 95 per cent confidence interval 1·36 to 6·49; P = 0·006), cirrhosis (OR 3·96, 1·94 to 8·09; P < 0·001) and surgical resection (OR 9·24, 4·68 to 18·25; P < 0·001) were significantly associated with worse HRQL (Table 3). Antiviral treatment (OR 0·44, 0·22 to 0·88; P = 0·021) was an independent protective factor for HRQL (Table 3).
Univariable and multivariable logistic regression analyses to identify predictors of health-related quality of life in patients who underwent percutaneous radiofrequency ablation or surgical resection for small hepatocellular carcinoma and with no recurrence
| . | . | . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|---|---|
| . | No. of patients . | No. with worse HRQL† . | Odds ratio* . | P . | Odds ratio* . | P . |
| Age (years) | 0·232 | |||||
| > 65 | 28 (11·4) | 17 (61) | 1·63 (0·73, 3·65) | |||
| ≤ 65 | 218 (88·6) | 106 (48·6) | 1·00 (reference) | |||
| Sex | 0·848 | |||||
| M | 215 (87·4) | 107 (49·8) | 0·93 (0·44, 1·97) | |||
| F | 31 (12·6) | 16 (52) | 1·00 (reference) | |||
| Marital status | 0·149 | |||||
| Single | 18 (7·3) | 12 (67) | 2·11 (0·77, 5·81) | |||
| Accompanied | 228 (92·7) | 111 (48·7) | 1·00 (reference) | |||
| Education (years) | 0·771 | |||||
| > 12 | 64 (26·0) | 33 (52) | 1·01 (0·62, 1·92) | |||
| ≤ 12 | 182 (74·0) | 90 (49·5) | 1·00 (reference) | |||
| Employment status | 0·102 | |||||
| Unemployed | 19 (7·7) | 13 (68) | 2·31 (0·85, 6·28) | |||
| Working or retired | 227 (92·3) | 110 (48·5) | 1·00 (reference) | |||
| Personal monthly income (RMB) | 0·372 | |||||
| < 5000 (< €586) | 119 (48·4) | 63 (52·9) | 1·26 (0·76, 2·07) | |||
| ≥ 5000 (≥ €586) | 127 (51·6) | 60 (47·2) | 1·00 (reference) | |||
| Concomitant diseases | 0·017 | 0·006 | ||||
| Yes | 47 (19·1) | 31 (66) | 2·25 (1·16, 4·38) | 2·97 (1·36, 6·49) | ||
| No | 199 (80·9) | 92 (46·2) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative HBV DNA (units/ml) | 0·145 | |||||
| < 200 | 157 (63·8) | 73 (46·5) | 1·48 (0·87, 2·49) | |||
| ≥ 200 | 89 (36·2) | 50 (56) | 1·00 (reference) | |||
| HBeAg positivity | 0·213 | |||||
| Yes | 52 (21·1) | 30 (58) | 1·48 (0·80, 2·75) | |||
| No | 194 (78·9) | 93 (47·9) | 1·00 (reference) | |||
| ALT (units/l) | 0·249 | |||||
| ≥ 40 | 111 (45·1) | 60 (54·1) | 1·35 (0·81, 2·23) | |||
| < 40 | 135 (54·9) | 63 (46·7) | 1·00 (reference) | |||
| Total bilirubin (µmol/l) | 0·172 | |||||
| ≥ 17 | 78 (31·7) | 44 (56) | 1·46 0·85, 2·50) | |||
| < 17 | 168 (68·3) | 79 (47·0) | 1·00 (reference) | |||
| Prothrombin time (s) | 0·153 | |||||
| ≥ 13 | 49 (19·9) | 29 (59) | 1·59 (0·84, 3·00) | |||
| < 13 | 197 (80·1) | 94 (47·7) | 1·00 (reference) | |||
| Albumin (g/l) | 0·056 | |||||
| < 35 | 30 (12·2) | 20 (67) | 2·19 (0·98, 4·91) | |||
| ≥ 35 | 216 (87·8) | 103 (47·7) | 1·00 (reference) | |||
| AFP (ng/ml) | 0·701 | |||||
| >100 | 135 (54·9) | 66 (48·9) | 0·91 (0·55, 1·50) | |||
| ≤ 100 | 111 (45·1) | 57 (51·4) | 1·00 (reference) | |||
| Cirrhosis | 0·001 | < 0·001 | ||||
| Yes | 187 (76·0) | 105 (56·1) | 2·92 (1·56, 5·45) | 3·96 (1·94, 8·09) | ||
| No | 59 (24·0) | 18 (31) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative TAE | 0·306 | |||||
| Yes | 16 (6·5) | 10 (63) | 1·73 (0·61, 4·91) | |||
| No | 230 (93·5) | 113 (49·1) | 1·00 (reference) | |||
| Antiviral treatment | 0·023 | 0·021 | ||||
| Yes | 55 (22·4) | 20 (36) | 0·49 (0·26, 0·91) | 0·44 (0·22, 0·88) | ||
| No | 191 (77·6) | 103 (53·9) | 1·00 (reference) | 1·00 (reference) | ||
| Therapeutic method | < 0·001 | < 0·001 | ||||
| Resection | 162 (65·9) | 105 (64·8) | 6·75 (3·66, 12·47) | 9·24 (4·68, 18·25) | ||
| RFA | 84 (34·1) | 18 (21) | 1·00 (reference) | 1·00 (reference) | ||
| . | . | . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|---|---|
| . | No. of patients . | No. with worse HRQL† . | Odds ratio* . | P . | Odds ratio* . | P . |
| Age (years) | 0·232 | |||||
| > 65 | 28 (11·4) | 17 (61) | 1·63 (0·73, 3·65) | |||
| ≤ 65 | 218 (88·6) | 106 (48·6) | 1·00 (reference) | |||
| Sex | 0·848 | |||||
| M | 215 (87·4) | 107 (49·8) | 0·93 (0·44, 1·97) | |||
| F | 31 (12·6) | 16 (52) | 1·00 (reference) | |||
| Marital status | 0·149 | |||||
| Single | 18 (7·3) | 12 (67) | 2·11 (0·77, 5·81) | |||
| Accompanied | 228 (92·7) | 111 (48·7) | 1·00 (reference) | |||
| Education (years) | 0·771 | |||||
| > 12 | 64 (26·0) | 33 (52) | 1·01 (0·62, 1·92) | |||
| ≤ 12 | 182 (74·0) | 90 (49·5) | 1·00 (reference) | |||
| Employment status | 0·102 | |||||
| Unemployed | 19 (7·7) | 13 (68) | 2·31 (0·85, 6·28) | |||
| Working or retired | 227 (92·3) | 110 (48·5) | 1·00 (reference) | |||
| Personal monthly income (RMB) | 0·372 | |||||
| < 5000 (< €586) | 119 (48·4) | 63 (52·9) | 1·26 (0·76, 2·07) | |||
| ≥ 5000 (≥ €586) | 127 (51·6) | 60 (47·2) | 1·00 (reference) | |||
| Concomitant diseases | 0·017 | 0·006 | ||||
| Yes | 47 (19·1) | 31 (66) | 2·25 (1·16, 4·38) | 2·97 (1·36, 6·49) | ||
| No | 199 (80·9) | 92 (46·2) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative HBV DNA (units/ml) | 0·145 | |||||
| < 200 | 157 (63·8) | 73 (46·5) | 1·48 (0·87, 2·49) | |||
| ≥ 200 | 89 (36·2) | 50 (56) | 1·00 (reference) | |||
| HBeAg positivity | 0·213 | |||||
| Yes | 52 (21·1) | 30 (58) | 1·48 (0·80, 2·75) | |||
| No | 194 (78·9) | 93 (47·9) | 1·00 (reference) | |||
| ALT (units/l) | 0·249 | |||||
| ≥ 40 | 111 (45·1) | 60 (54·1) | 1·35 (0·81, 2·23) | |||
| < 40 | 135 (54·9) | 63 (46·7) | 1·00 (reference) | |||
| Total bilirubin (µmol/l) | 0·172 | |||||
| ≥ 17 | 78 (31·7) | 44 (56) | 1·46 0·85, 2·50) | |||
| < 17 | 168 (68·3) | 79 (47·0) | 1·00 (reference) | |||
| Prothrombin time (s) | 0·153 | |||||
| ≥ 13 | 49 (19·9) | 29 (59) | 1·59 (0·84, 3·00) | |||
| < 13 | 197 (80·1) | 94 (47·7) | 1·00 (reference) | |||
| Albumin (g/l) | 0·056 | |||||
| < 35 | 30 (12·2) | 20 (67) | 2·19 (0·98, 4·91) | |||
| ≥ 35 | 216 (87·8) | 103 (47·7) | 1·00 (reference) | |||
| AFP (ng/ml) | 0·701 | |||||
| >100 | 135 (54·9) | 66 (48·9) | 0·91 (0·55, 1·50) | |||
| ≤ 100 | 111 (45·1) | 57 (51·4) | 1·00 (reference) | |||
| Cirrhosis | 0·001 | < 0·001 | ||||
| Yes | 187 (76·0) | 105 (56·1) | 2·92 (1·56, 5·45) | 3·96 (1·94, 8·09) | ||
| No | 59 (24·0) | 18 (31) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative TAE | 0·306 | |||||
| Yes | 16 (6·5) | 10 (63) | 1·73 (0·61, 4·91) | |||
| No | 230 (93·5) | 113 (49·1) | 1·00 (reference) | |||
| Antiviral treatment | 0·023 | 0·021 | ||||
| Yes | 55 (22·4) | 20 (36) | 0·49 (0·26, 0·91) | 0·44 (0·22, 0·88) | ||
| No | 191 (77·6) | 103 (53·9) | 1·00 (reference) | 1·00 (reference) | ||
| Therapeutic method | < 0·001 | < 0·001 | ||||
| Resection | 162 (65·9) | 105 (64·8) | 6·75 (3·66, 12·47) | 9·24 (4·68, 18·25) | ||
| RFA | 84 (34·1) | 18 (21) | 1·00 (reference) | 1·00 (reference) | ||
Values in parentheses are percentages unless indicated otherwise;
values in parentheses are 95 per cent confidence intervals.
Worse health-related quality of life (HRQL) was defined as a change in Functional Assessment of Cancer Therapy – Hepatobiliary (FACT-Hep) total score below the median value of 128. RMB, renminbi; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; AFP, α-fetoprotein; TAE, transarterial embolization; RFA, radiofrequency ablation.
Univariable and multivariable logistic regression analyses to identify predictors of health-related quality of life in patients who underwent percutaneous radiofrequency ablation or surgical resection for small hepatocellular carcinoma and with no recurrence
| . | . | . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|---|---|
| . | No. of patients . | No. with worse HRQL† . | Odds ratio* . | P . | Odds ratio* . | P . |
| Age (years) | 0·232 | |||||
| > 65 | 28 (11·4) | 17 (61) | 1·63 (0·73, 3·65) | |||
| ≤ 65 | 218 (88·6) | 106 (48·6) | 1·00 (reference) | |||
| Sex | 0·848 | |||||
| M | 215 (87·4) | 107 (49·8) | 0·93 (0·44, 1·97) | |||
| F | 31 (12·6) | 16 (52) | 1·00 (reference) | |||
| Marital status | 0·149 | |||||
| Single | 18 (7·3) | 12 (67) | 2·11 (0·77, 5·81) | |||
| Accompanied | 228 (92·7) | 111 (48·7) | 1·00 (reference) | |||
| Education (years) | 0·771 | |||||
| > 12 | 64 (26·0) | 33 (52) | 1·01 (0·62, 1·92) | |||
| ≤ 12 | 182 (74·0) | 90 (49·5) | 1·00 (reference) | |||
| Employment status | 0·102 | |||||
| Unemployed | 19 (7·7) | 13 (68) | 2·31 (0·85, 6·28) | |||
| Working or retired | 227 (92·3) | 110 (48·5) | 1·00 (reference) | |||
| Personal monthly income (RMB) | 0·372 | |||||
| < 5000 (< €586) | 119 (48·4) | 63 (52·9) | 1·26 (0·76, 2·07) | |||
| ≥ 5000 (≥ €586) | 127 (51·6) | 60 (47·2) | 1·00 (reference) | |||
| Concomitant diseases | 0·017 | 0·006 | ||||
| Yes | 47 (19·1) | 31 (66) | 2·25 (1·16, 4·38) | 2·97 (1·36, 6·49) | ||
| No | 199 (80·9) | 92 (46·2) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative HBV DNA (units/ml) | 0·145 | |||||
| < 200 | 157 (63·8) | 73 (46·5) | 1·48 (0·87, 2·49) | |||
| ≥ 200 | 89 (36·2) | 50 (56) | 1·00 (reference) | |||
| HBeAg positivity | 0·213 | |||||
| Yes | 52 (21·1) | 30 (58) | 1·48 (0·80, 2·75) | |||
| No | 194 (78·9) | 93 (47·9) | 1·00 (reference) | |||
| ALT (units/l) | 0·249 | |||||
| ≥ 40 | 111 (45·1) | 60 (54·1) | 1·35 (0·81, 2·23) | |||
| < 40 | 135 (54·9) | 63 (46·7) | 1·00 (reference) | |||
| Total bilirubin (µmol/l) | 0·172 | |||||
| ≥ 17 | 78 (31·7) | 44 (56) | 1·46 0·85, 2·50) | |||
| < 17 | 168 (68·3) | 79 (47·0) | 1·00 (reference) | |||
| Prothrombin time (s) | 0·153 | |||||
| ≥ 13 | 49 (19·9) | 29 (59) | 1·59 (0·84, 3·00) | |||
| < 13 | 197 (80·1) | 94 (47·7) | 1·00 (reference) | |||
| Albumin (g/l) | 0·056 | |||||
| < 35 | 30 (12·2) | 20 (67) | 2·19 (0·98, 4·91) | |||
| ≥ 35 | 216 (87·8) | 103 (47·7) | 1·00 (reference) | |||
| AFP (ng/ml) | 0·701 | |||||
| >100 | 135 (54·9) | 66 (48·9) | 0·91 (0·55, 1·50) | |||
| ≤ 100 | 111 (45·1) | 57 (51·4) | 1·00 (reference) | |||
| Cirrhosis | 0·001 | < 0·001 | ||||
| Yes | 187 (76·0) | 105 (56·1) | 2·92 (1·56, 5·45) | 3·96 (1·94, 8·09) | ||
| No | 59 (24·0) | 18 (31) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative TAE | 0·306 | |||||
| Yes | 16 (6·5) | 10 (63) | 1·73 (0·61, 4·91) | |||
| No | 230 (93·5) | 113 (49·1) | 1·00 (reference) | |||
| Antiviral treatment | 0·023 | 0·021 | ||||
| Yes | 55 (22·4) | 20 (36) | 0·49 (0·26, 0·91) | 0·44 (0·22, 0·88) | ||
| No | 191 (77·6) | 103 (53·9) | 1·00 (reference) | 1·00 (reference) | ||
| Therapeutic method | < 0·001 | < 0·001 | ||||
| Resection | 162 (65·9) | 105 (64·8) | 6·75 (3·66, 12·47) | 9·24 (4·68, 18·25) | ||
| RFA | 84 (34·1) | 18 (21) | 1·00 (reference) | 1·00 (reference) | ||
| . | . | . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|---|---|
| . | No. of patients . | No. with worse HRQL† . | Odds ratio* . | P . | Odds ratio* . | P . |
| Age (years) | 0·232 | |||||
| > 65 | 28 (11·4) | 17 (61) | 1·63 (0·73, 3·65) | |||
| ≤ 65 | 218 (88·6) | 106 (48·6) | 1·00 (reference) | |||
| Sex | 0·848 | |||||
| M | 215 (87·4) | 107 (49·8) | 0·93 (0·44, 1·97) | |||
| F | 31 (12·6) | 16 (52) | 1·00 (reference) | |||
| Marital status | 0·149 | |||||
| Single | 18 (7·3) | 12 (67) | 2·11 (0·77, 5·81) | |||
| Accompanied | 228 (92·7) | 111 (48·7) | 1·00 (reference) | |||
| Education (years) | 0·771 | |||||
| > 12 | 64 (26·0) | 33 (52) | 1·01 (0·62, 1·92) | |||
| ≤ 12 | 182 (74·0) | 90 (49·5) | 1·00 (reference) | |||
| Employment status | 0·102 | |||||
| Unemployed | 19 (7·7) | 13 (68) | 2·31 (0·85, 6·28) | |||
| Working or retired | 227 (92·3) | 110 (48·5) | 1·00 (reference) | |||
| Personal monthly income (RMB) | 0·372 | |||||
| < 5000 (< €586) | 119 (48·4) | 63 (52·9) | 1·26 (0·76, 2·07) | |||
| ≥ 5000 (≥ €586) | 127 (51·6) | 60 (47·2) | 1·00 (reference) | |||
| Concomitant diseases | 0·017 | 0·006 | ||||
| Yes | 47 (19·1) | 31 (66) | 2·25 (1·16, 4·38) | 2·97 (1·36, 6·49) | ||
| No | 199 (80·9) | 92 (46·2) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative HBV DNA (units/ml) | 0·145 | |||||
| < 200 | 157 (63·8) | 73 (46·5) | 1·48 (0·87, 2·49) | |||
| ≥ 200 | 89 (36·2) | 50 (56) | 1·00 (reference) | |||
| HBeAg positivity | 0·213 | |||||
| Yes | 52 (21·1) | 30 (58) | 1·48 (0·80, 2·75) | |||
| No | 194 (78·9) | 93 (47·9) | 1·00 (reference) | |||
| ALT (units/l) | 0·249 | |||||
| ≥ 40 | 111 (45·1) | 60 (54·1) | 1·35 (0·81, 2·23) | |||
| < 40 | 135 (54·9) | 63 (46·7) | 1·00 (reference) | |||
| Total bilirubin (µmol/l) | 0·172 | |||||
| ≥ 17 | 78 (31·7) | 44 (56) | 1·46 0·85, 2·50) | |||
| < 17 | 168 (68·3) | 79 (47·0) | 1·00 (reference) | |||
| Prothrombin time (s) | 0·153 | |||||
| ≥ 13 | 49 (19·9) | 29 (59) | 1·59 (0·84, 3·00) | |||
| < 13 | 197 (80·1) | 94 (47·7) | 1·00 (reference) | |||
| Albumin (g/l) | 0·056 | |||||
| < 35 | 30 (12·2) | 20 (67) | 2·19 (0·98, 4·91) | |||
| ≥ 35 | 216 (87·8) | 103 (47·7) | 1·00 (reference) | |||
| AFP (ng/ml) | 0·701 | |||||
| >100 | 135 (54·9) | 66 (48·9) | 0·91 (0·55, 1·50) | |||
| ≤ 100 | 111 (45·1) | 57 (51·4) | 1·00 (reference) | |||
| Cirrhosis | 0·001 | < 0·001 | ||||
| Yes | 187 (76·0) | 105 (56·1) | 2·92 (1·56, 5·45) | 3·96 (1·94, 8·09) | ||
| No | 59 (24·0) | 18 (31) | 1·00 (reference) | 1·00 (reference) | ||
| Preoperative TAE | 0·306 | |||||
| Yes | 16 (6·5) | 10 (63) | 1·73 (0·61, 4·91) | |||
| No | 230 (93·5) | 113 (49·1) | 1·00 (reference) | |||
| Antiviral treatment | 0·023 | 0·021 | ||||
| Yes | 55 (22·4) | 20 (36) | 0·49 (0·26, 0·91) | 0·44 (0·22, 0·88) | ||
| No | 191 (77·6) | 103 (53·9) | 1·00 (reference) | 1·00 (reference) | ||
| Therapeutic method | < 0·001 | < 0·001 | ||||
| Resection | 162 (65·9) | 105 (64·8) | 6·75 (3·66, 12·47) | 9·24 (4·68, 18·25) | ||
| RFA | 84 (34·1) | 18 (21) | 1·00 (reference) | 1·00 (reference) | ||
Values in parentheses are percentages unless indicated otherwise;
values in parentheses are 95 per cent confidence intervals.
Worse health-related quality of life (HRQL) was defined as a change in Functional Assessment of Cancer Therapy – Hepatobiliary (FACT-Hep) total score below the median value of 128. RMB, renminbi; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; AFP, α-fetoprotein; TAE, transarterial embolization; RFA, radiofrequency ablation.
Discussion
In this study, patients who underwent percutaneous RFA for small HCC had better HRQL than those treated by surgical resection. There did not appear to be a difference in overall or disease-free survival between groups, in keeping with the results of earlier trials25,26. Percutaneous RFA has become increasingly popular in the treatment of solitary, small HCC4. Livraghi and colleagues27 even advocated percutaneous RFA as a first-line treatment for very early HCCs (2 cm or smaller), which may seem justified against the background of the present study. The present analysis included only patients who could be cured by either surgical resection or percutaneous RFA, with Child–Pugh class A liver function, and a solitary, small (maximum 3 cm) HCC as HRQL could be compromised by residual/recurrent tumour and by pretreatment liver function13. Importantly, baseline sociodemographic and medical characteristics were comparable in the two groups.
Surgery has considerable short-term effects on HRQL. Similar to previous studies, there was a significantly greater reduction in physical, functional and hepatobiliary cancer subscale scores as well as in the FACT-Hep total score shortly after surgical treatment than after percutaneous RFA. There was marked improvement in HRQL 3–6 months after surgery. The percutaneous RFA group showed a considerably smaller drop in these subscale scores shortly after treatment, and this was followed by a steady improvement in HRQL from 3 months to the end of the study.
It is not surprising that HRQL scores were lower in the surgical resection group shortly after treatment. First, patients who underwent surgical resection experienced more trauma, post-treatment morbidity and worse liver function than those treated by percutaneous RFA. Thus, the worsened HRQL was possibly due to the direct consequences of surgery, especially in the early postoperative period. In previous studies13,28, surgery-related complications and impaired liver function affected physical, psychological and social well-being, and caused considerable HRQL impairment. Second, compared with surgical resection, percutaneous RFA had the advantages of being less traumatic, causing fewer post-treatment complications, and it was associated with earlier recovery. Previous studies29 have shown little change in liver function after percutaneous RFA. The combination of fewer complications and less liver damage may have contributed to the higher HRQL scores shortly after percutaneous RFA.
The effects of surgical resection on HRQL were not restricted to the short term. The difference in HRQL scores persisted for up to 36 months after treatment, although the gap narrowed gradually. Even though the effects on HRQL appeared to be more pronounced in the short term after surgical resection, many patients felt incisional pain and were incapable of heavier physical labour in the long term. Recent studies30,31 have shown that incisional pain, impaired wound healing and social isolation owing to the underlying disease affect HRQL after surgical resection for liver malignancy. The present study not only supported the previous findings, but also showed that surgical resection was an independent risk factor for poor post-treatment HRQL. Antiviral therapy improved HRQL of patients with viral hepatitis probably because of improvements in liver fibrosis and preservation of liver function9.
The limitations of the study are related to its non-randomized design. At the time this study was carried out, percutaneous RFA was still a relatively new, potentially curative therapy, which was not accepted by some patients. Thus, the number of patients prepared to undergo surgical resection was twice the number willing to accept percutaneous RFA. It would have been difficult to recruit enough patients into a randomized clinical trial. Another potential bias of this study is that patient personality type might have affected the choice of treatment, and at the same time the HRQL score. In addition, only the FACT-Hep was used to assess HRQL in this study. The European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 has also been used widely as an instrument for assessing HRQL in patients with cancer. However, a drawback of the EORTC QLQ-30 may be that it does not consider differences between cancer types.
Acknowledgements
G.H. and X.C. contributed equally to this work. The study was funded by the State Key Project on Infectious Diseases of China (2012ZX10002010, 2012ZX10002016), the Science Fund for Creative Research Groups, China (81201940) and the National Science Foundation of China (81201555).
Disclosure: The authors declare no conflict of interest.