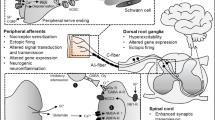
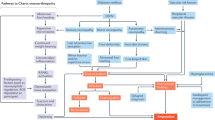
Abstract
Treatment of neuropathic pain in general, and in diabetic neuropathy in particular, often results in inadequate pain relief, even with first-line drugs. New therapeutic approaches are therefore mechanism-based targeting the individual pathophysiological pain mechanisms. The sensory phenotype of a patient, i.e., the sensory signs and symptoms, can provide indirect information about these underlying pathomechanisms. Quantitative sensory testing (QST) can be used to assess the function of the large and small nerve fibers or the corresponding central pathways to generate an individual sensory profile with loss and gain of function parameters for each patient. Stratification of patients according to these profiles can help to identify subgroups of patients with similar pathophysiological mechanisms. In diabetic neuropathy the most frequent subgroup is dominated by a loss of small and large fiber function with little or no gain of function (deafferentation/sensory loss cluster). A similar approach relates to individual patient symptoms using patient reported outcome measures, i.e., neuropathic pain specific questionnaires. This phenotypic-based subgrouping has proven to be a promising approach to prospectively identify responders to a certain therapy, potentially realizing the concept of a new, individualized mechanism-based pain therapy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sloan G, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract. 2018;144:177–91. https://doi.org/10.1016/j.diabres.2018.08.020.
Attal N, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113. https://doi.org/10.1111/j.1468-1331.2010.02999.x.
Finnerup NB, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. https://doi.org/10.1016/S1474-4422(14)70251-0.
Bril V, et al. Evidence-based guideline: treatment of painful diabetic neuropathy–report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve. 2011;43(6):910–7. https://doi.org/10.1002/mus.22092.
Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21(9):976–82. https://doi.org/10.1111/j.1464-5491.2004.01271.x.
von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73(4):638–52. https://doi.org/10.1016/j.neuron.2012.02.008.
LaMotte RH, Thalhammer JG, Torebjörk HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982;2(6):765–81.
LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66(1):190–211. https://doi.org/10.1152/jn.1991.66.1.190.
Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20(4):355–61. https://doi.org/10.1016/0304-3959(84)90112-X.
Yarnitsky D, Ochoa JL. Differential effect of compression-ischaemia block on warm sensation and heat-induced pain. Brain. 1991;114(2):907–13. https://doi.org/10.1093/brain/114.2.907.
Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122(12):2245–57. https://doi.org/10.1093/brain/122.12.2245.
Geber C, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain. 2011;152(3):548–56. https://doi.org/10.1016/j.pain.2010.11.013.
Vollert J, et al. Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. Pain. 2016;157(3):750–8. https://doi.org/10.1097/j.pain.0000000000000433.
Rolke R, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–43. https://doi.org/10.1016/j.pain.2006.01.041.
Rolke R, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77–88. https://doi.org/10.1016/j.ejpain.2005.02.003.
Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–50. https://doi.org/10.1016/j.pain.2010.05.002.
Treede R-D. Chapter 1 pain and hyperalgesia: definitions and theories. Handb Clin Neurol. 2006;81:3–10. https://doi.org/10.1016/S0072-9752(06)80005-9.
Obata K, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115(9):2393–401. https://doi.org/10.1172/JCI25437.
Fruhstorfer H, Gross W, Selbmann O. von Frey hairs: new materials for a new design. Eur J Pain. 2001;5(3):341–2. https://doi.org/10.1053/eujp.2001.0250.
Herrero JF, Laird JM, López-García JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. https://doi.org/10.1016/s0301-0082(99)00051-9.
Glass GV, Stanley JX. Statistical methods in education and psychology. Englewood Cliffs: Prentice-Hall; 1970.
Blankenburg M, et al. Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain. 2010;149(1):76–88. https://doi.org/10.1016/j.pain.2010.01.011.
Pfau DB, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain. 2014;155(5):1002–15. https://doi.org/10.1016/j.pain.2014.02.004.
Gierthmühlen J, et al. Who is healthy? Aspects to consider when including healthy volunteers in QST--based studies-a consensus statement by the EUROPAIN and NEUROPAIN consortia. Pain. 2015;156(11):2203–11. https://doi.org/10.1097/j.pain.0000000000000227.
Smith SM, et al. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain. 2017;18(7):757–77. https://doi.org/10.1016/j.jpain.2017.02.429.
Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11(11):999–1005. https://doi.org/10.1016/S1474-4422(12)70189-8.
Blankenburg M, et al. Childhood diabetic neuropathy: functional impairment and non-invasive screening assessment. Diabet Med. 2012;29(11):1425–32. https://doi.org/10.1111/j.1464-5491.2012.03685.x.
Shillo P, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep. 2019;19(6):32. https://doi.org/10.1007/s11892-019-1150-5.
Krämer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004;27(10):2386–91. https://doi.org/10.2337/diacare.27.10.2386.
Themistocleous AC, et al. The pain in neuropathy study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157(5):1132–45. https://doi.org/10.1097/j.pain.0000000000000491.
Raputova J, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain. 2017;158(12):2340–53. https://doi.org/10.1097/j.pain.0000000000001034.
Baron R, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–72. https://doi.org/10.1097/j.pain.0000000000000753.
Vollert J, et al. Pathophysiological mechanisms of neuropathic pain: comparison of sensory phenotypes in patients and human surrogate pain models. Pain. 2018;159(6):1090–102. https://doi.org/10.1097/j.pain.0000000000001190.
Vollert J, et al. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. Pain. 2017;158(8):1446–55. https://doi.org/10.1097/j.pain.0000000000000935.
Buliteanu A, et al. Validation of a bedside quantitative sensory testing (QST) protocol in chronic neuropathic pain. J Pain. 2018;19(3):S52. https://doi.org/10.1016/j.jpain.2017.12.123.
Zhu GC, et al. Concurrent validity of a low-cost and time-efficient clinical sensory test battery to evaluate somatosensory dysfunction. Eur J Pain. 2019;23(10):1826–38. https://doi.org/10.1002/ejp.1456.
Reimer M, et al. Sensory bedside testing: a simple stratification approach for sensory phenotyping. Pain Rep. 2020;5(3):e820. https://doi.org/10.1097/PR9.0000000000000820.
Demant DT, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155(11):2263–73. https://doi.org/10.1016/j.pain.2014.08.014.
Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5(4):209–27. https://doi.org/10.1006/nbdi.1998.0204.
Suh Y-G, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des. 2005;11(21):2687–98. https://doi.org/10.2174/1381612054546789.
Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7(1):3–12. https://doi.org/10.1016/j.jpain.2005.09.006.
Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010;33:325–47. https://doi.org/10.1146/annurev-neuro-060909-153234.
Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev. 2014;94(1):81–140. https://doi.org/10.1152/physrev.00023.2013.
Tandon M et al. A simple algorithm to identify likely responders to GRC 17356 in patients of painful diabetic peripheral neuropathy using sensory mapping
de Araujo DSM, Nassini R, Geppetti P, De Logu F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets. 2020;24(10):997–1008. https://doi.org/10.1080/14728222.2020.1815191.
Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–8. https://doi.org/10.1016/j.pain.2012.02.021.
Sachau J, Baron R. Neuropathic pain therapy: a puzzle of different approaches to stratify patients. Pain. 2020. https://doi.org/10.1097/j.pain.0000000000002120
Freynhagen R, Baron R, Gockel U, Tölle TR. Pain detect: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–20. https://doi.org/10.1185/030079906X132488.
Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138(2):343–53. https://doi.org/10.1016/j.pain.2008.01.006.
Scholz J, et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6(4):e1000047. https://doi.org/10.1371/journal.pmed.1000047.
Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. Pain. 2009;146(1):34–40. https://doi.org/10.1016/j.pain.2009.06.001.
Bouhassira D, et al. Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108(3):248–57. https://doi.org/10.1016/j.pain.2003.12.024.
Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two? Curr Diab Rep. 2013;13:7. https://doi.org/10.1007/s11892-013-0387-7.
Tölle TR, et al. Pain predict: first interim data from the development of a new patient-reported pain questionnaire to predict treatment response using sensory symptom profiles. Curr Med Res Opin. 2019;35(7):1177–85. https://doi.org/10.1080/03007995.2018.1562687.
Rice ASC, Finnerup NB, Kemp HI, Currie GL, Baron R. Sensory profiling in animal models of neuropathic pain: a call for back-translation. Pain. 2018;159(5):819–24. https://doi.org/10.1097/j.pain.0000000000001138.
Sveen KA, et al. Small- and large-fiber neuropathy after 40 years of type 1 diabetes: associations with glycemic control and advanced protein glycation: the Oslo Study. Diabetes Care. 2013;36(11):3712–7. https://doi.org/10.2337/dc13-0788.
Goyal SN, et al. Challenges and issues with streptozotocin-induced diabetes - a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact. 2016;244:49–63. https://doi.org/10.1016/j.cbi.2015.11.032.
Klinck MP, et al. Translational pain assessment: could natural animal models be the missing link? Pain. 2017;158(9):1633–46. https://doi.org/10.1097/j.pain.0000000000000978.
Bouhassira D, et al. Stratification of patients based on the neuropathic pain symptom inventory: development and validation of a new algorithm. Pain. 2020. https://doi.org/10.1097/j.pain.0000000000002130
Acknowledgement
We would like to thank Dilara Kersebaum for her excellent help in language editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sachau, J., Sendel, M., Baron, R. (2023). Sensory Profiles and Diabetic Neuropathy. In: Tesfaye, S., Gibbons, C.H., Malik, R.A., Veves, A. (eds) Diabetic Neuropathy. Contemporary Diabetes. Humana, Cham. https://doi.org/10.1007/978-3-031-15613-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-15613-7_7
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-031-15612-0
Online ISBN: 978-3-031-15613-7
eBook Packages: MedicineMedicine (R0)