Abstract
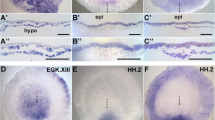
The neural crest (NC) is usually defined as a cell type arising at the border of the neural plate and the epidermis in vertebrate embryos. While accurate, this definition implies that the border exists as a distinct boundary, which is not really the case. Like other domains in the early embryo, the NC is not a sharply delimited territory, but rather is an overlapping zone of specification that has characteristics of both epidermis and neural plate, with additional characteristics of its own. This can be seen in the spatial pattern of regulatory factors that have been implicated in NC induction, many of which are shared.1 For example Msx1 and AP2 are also expressed in the epidermis, at lower levels, and c-myc is likewise transcribed in neural plate in addition to NC. Nor is this limited to regulatory factors. The epidermal keratins, which constitute the intermediate filament cytoskeleton of epidermal cells are also expressed in NC, at a lower level than the epidermis. This can be seen by in situ hybridization (Fig. 1A) as a region of relatively weak but significant signal in the cranial neural crest region. Another indication of the fuzzy nature of NC comes from lineage mapping experiments in the chick embryo, which show that cells fated to differentiate as NC, neural plate, epidermis and placodal derivatives are all intermingled in the neural-epidermal boundary region.2 Nor is there always a clear gap visible between neural plate and epidermal gene expression domains, for example in a double in situ with epidermal keratin and the pan-neural marker NCAM the two domains are contiguous (Fig. 1B). Expression of keratin genes is also evident in animal caps that have been dissected from embryos injected with BMP antagonists, such as chordin, along with a canonical Wnt signal molecule, such as Wnt3a.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Developmental Biology 2004; 275(1):1–11.
Streit A. Extensive cell movements accompany formation of the otic placode. Dev Biol 2002; 249(2):237–254.
Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Developmental Cell 2004; 7(3):291–299.
Cohen SM, Bronner G, Kuttner F et al. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature 1989; 338(6214):432–434.
Bendall AJ, Abate-Shen C. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene 2000; 247(1–2):17–31.
Feledy JA, Morasso MI, Jang SI et al. Transcriptional activation by the homeodomain protein distal-less 3. Nucleic Acids Res 1999; 27(3):764–770.
Stock DW, Ellies DL, Zhao Z et al. The evolution of the vertebrate Dlx gene family. Proc Natl Acad Sci USA 1996; 93(20):10858–10863.
Luo T, Matsuo-Takasaki M, Lim JH et al. Differential regulation of Dlx gene expression by a BMP morphogenetic gradient. Int J Dev Biol 2001; 45(4):681–684.
Luo T, Matsuo-Takasaki M, Sargent TD. Distinct roles for Distal-less genes Dlx3 and Dlx5 in regulating ectodermal development in Xenopus. Mol Reprod Dev 2001; 60(3):331–337.
Papalopulu N, Kintner C. Xenopus Distal-less related homeobox genes are expressed in the developing forebrain and are induced by planar signals. Development 1993; 117(3):961–975.
Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development 2002; 129(19):4371–4386.
Zhang H, Catron KM, Abate-Shen C. A role for the Msx-1 homeodomain in transcriptional regulation: Residues in the N-terminal arm mediate TATA binding protein interaction and transcriptional repression. Proc Natl Acad Sci USA 1996; 93(5):1764–1769.
Lee H, Habas R, Abate-Shen C. MSX1 cooperates with histone H1b for inhibition of transcription and myogenesis. Science 2004; 304(5677):1675–1678.
Zhang H, Hu G, Wang H et al. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol Cell Biol 1997; 17(5):2920–2932.
Bryan JT, Morasso MI. The Dlx3 protein harbors basic residues required for nuclear localization, transcriptional activity and binding to Msx1. J Cell Sci 2000; 113 (Pt 22):4013–4023.
Alvarez Martinez CE, Binato R, Gonzalez S et al. Characterization of a Smad motif similar to Drosophila mad in the mouse Msx 1 promoter. Biochem Biophys Res Commun 2002; 291(3):655–662.
Suzuki A, Ueno N, Hemmati-Brivanlou A. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 1997; 124(16):3037–3044.
Brugger SM, Merrill AE, Torres-Vazquez J et al. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development 2004; 131(20):5153–5165.
Jaynes JB, O’Farrell PH. Active repression of transcription by the engrailed homeodomain protein. EMBO J 1991; 10(6):1427–1433.
Miyama K, Yamada G, Yamamoto TS et al. A BMP-inducible gene, dlx5, regulates osteoblast differentiation and mesoderm induction. Dev Biol 1999; 208(1):123–133.
Feledy JA, Beanan MJ, Sandoval JJ et al. Inhibitory patterning of the anterior neural plate in Xenopus by homeodomain factors Dlx3 and Msx1. Dev Biol 1999; 212(2):455–464.
Beanan MJ, Feledy JA, Sargent TD. Regulation of early expression of Dlx3, a Xenopus anti-neural factor, by beta-catenin signaling. Mech Dev 2000; 91(1–2):227–235.
Woda JM, Pastagia J, Mercola M et al. Dlx proteins position the neural plate border and determine adjacent cell fates. Development 2003; 130(2):331–342.
Saint-Germain N, Lee YH, Zhang YH et al. Specification of the otic placode depends on Sox9 function in Xenopus. Development 2004; 131(8):1755–1763.
Solomon KS, Fritz A. Concerted action of two dlx paralogs in sensory placode formation. Development 2002; 129(13):3127–3136.
McLarren KW, Litsiou A, Streit A. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev Biol 2003; 259(1):34–47.
Kolm PJ, Sive HL. Efficient hormone-inducible protein function in Xenopus laevis. Dev Biol 1995; 171(1):267–272.
Kaji T, Artinger KB. dlx3b and dlx4b function in the development of Rohon-Beard sensory neurons and trigeminal placode in the zebrafish neurula. Dev Biol 2004; 276(2):523–540.
Tribulo C, Aybar MJ, Nguyen VH et al. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 2003; 130(26):6441–6452.
Mayor R, Young R, Vargas A. Development of neural crest in Xenopus. Curr Top Dev Biol 1999; 43:85–113.
Willert J, Epping M, Pollack JR et al. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2002; 2(1):8.
Tribulo C, Aybar MJ, Sanchez SS et al. A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of the neural crest. Dev Biol 2004; 275(2):325–342.
Mohibullah N, Donner A, Ippolito JA et al. SELEX and missing phosphate contact analyses reveal flexibility within the AP-2[alpha] protein: DNA binding complex. Nucleic Acids Res 1999; 27(13):2760–2769.
Satoda M, Zhao F, Diaz GA et al. Mutations in TFAP2B cause Char syndrome, a familial form of patent ductus arteriosus. Nat Genet 2000; 25(1):42–46.
Hilger-Eversheim K, Moser M, Schorle H et al. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene 2000; 260(1–2): 1–12.
Luo T, Matsuo-Takasaki M, Thomas ML et al. Transcription factor AP-2 is an essential and direct regulator of epidermal development in Xenopus. Dev Biol 2002; 245(1):136–144.
Maconochie M, Krishnamurthy R, Nonchev S et al. Regulation of Hoxa2 in cranial neural crest cells involves members of the AP-2 family. Development 1999; 126(7):1483–1494.
Knight RD, Javidan Y, Nelson S et al. Skeletal and pigment cell defects in the lockjaw mutant reveal multiple roles for zebrafish tfap2a in neural crest development. Dev Dyn 2004; 229(1):87–98.
Mitchell PJ, Timmons PM, Hebert JM et al. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev 1991; 5(1):105–119.
Schorle H, Meier P, Buchert M et al. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 1996; 381(6579):235–238.
Zhang J, Hagopian-Donaldson S, Serbedzija G et al. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 1996; 381(6579):238–241.
Moser M, Pscherer A, Roth C et al. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev 1997; 11(15):1938–1948.
Auman HJ, Nottoli T, Lakiza O et al. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development 2002; 129(11):2733–2747.
Brewer S, Jiang X, Donaldson S et al. Requirement for AP-2alpha in cardiac outflow tract morphogenesis. Mech Dev 2002; 110(1–2):139–149.
Brewer S, Feng W, Huang J et al. Wnt1-Cremediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev Biol 2004; 267(1):135–152.
Danielian PS, Muccino D, Rowitch DH et al. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 1998; 8(24):1323–1326.
Neuhauss SC, Solnica-Krezel L, Schier AF et al. Mutations affecting craniofacial development in zebrafish. Development 1996; 123:357–367.
Schilling TF, Piotrowski T, Grandel H et al. Jaw and branchial arch mutants in zebrafish I: Branchial arches. Development 1996; 123:329–344.
Barrallo-Gimeno A, Holzschuh J, Driever W et al. Neural crest survival and differentiation in zebrafish depends on mont blanc/tfap2a gene function. Development 2004; 131(7):1463–1477.
Knight RD, Nair S, Nelson SS et al. lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development 2003; 130(23):5755–5768.
Luo T, Lee YH, Saint-Jeannet JP et al. Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc Natl Acad Sci USA 2003; 100(2):532–537.
Dagle JM, Littig JL, Sutherland LB et al. Targeted elimination of zygotic messages in Xenopus laevis embryos by modified oligpnucleotides possessing terminal cationic linkages. Nucleic Acids Res 2000; 28(10):2153–2157.
Dagle JM, Weeks DL. Selective degradation of targeted mRNAs using partially modified oligonucleotides. Methods Enzymol 2000; 313:420–436.
Saint-Jeannet JP, He X, Varmus HE et al. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc Natl Acad Sci USA 1997; 94(25):13713–13718.
LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: Evidence for a two-signal model. Development 1998; 125(13):2403–2414.
Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development 2003; 130(14):3111–3124.
Vallin J, Thuret R, Giacomello E et al. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J Biol Chem 2001; 276(32):30350–30358.
Locascio A, Manzanares M, Blanco MJ et al. Modularity and reshuffling of Snail and Slug expression during vertebrate evolution. Proc Nad Acad Sci USA 2002; 99(26):16841–16846.
Satokata I, Ma L, Ohshima H et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 2000; 24(4):391–395.
Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 1994; 6(4):348–356.
Kishi M, Mizuseki K, Sasai N et al. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development 2000; 127(4):791–800.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2006 Landes Bioscience and Springer Science+Business Media
About this chapter
Cite this chapter
Sargent, T.D. (2006). Transcriptional Regulation at the Neural Plate Border. In: Saint-Jeannet, JP. (eds) Neural Crest Induction and Differentiation. Advances in Experimental Medicine and Biology, vol 589. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-46954-6_3
Download citation
DOI: https://doi.org/10.1007/978-0-387-46954-6_3
Publisher Name: Springer, Boston, MA
Print ISBN: 978-0-387-35136-0
Online ISBN: 978-0-387-46954-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)