Abstract
The present study is concerned with the in vitro study of different sized titanium dioxide (TiO2) nanoparticles' (NPs) penetration and accumulation in human normal lung (NL) tissue and lung adenocarcinoma tumor (LAT) tissue by the methods of continuous optical coherence tomography (OCT) monitoring and diffuse reflectance (DR) spectra measurement, and their evaluating the effects of TiO2 NPs in two sizes (60 nm and 100 nm) and their combination with ultrasound (US) on the optical properties of human NL and LAT tissue. Spectral measurements indicate that TiO2 NPs penetrate and accumulate into the tissues and thus induce enhancement of DR. The averaged and normalized OCT signal intensity suggests that light penetration depth is significantly enlarged by ultrasound. The average attenuation coefficient of NL tissue changes from 5.10 ± 0.26 mm−1 to 3.12 ± 0.43 mm−1 and 2.15 ± 0.54 mm−1 at 120 min for 60 nm TiO2 NPs and 60 nm TiO2NPs/US treatment, respectively, and from 5.54 ± 0.46 mm−1 to 3.24 ± 0.73 mm−1 and 2.69 ± 0.34 mm−1 at 150 min for 100 nm TiO2 NPs and 100 nm TiO2NPs/US, respectively. The average attenuation coefficient of LAT tissue changes from 9.12 ± 0.54 mm−1 to 4.54 ± 0.39 mm−1 and 3.61 ± 0.38 mm−1 at 120 min for 60 nm TiO2 NPs and 60 nm TiO2NPs/US treatment, respectively, and from 9.79 ± 0.32 mm−1 to 5.12 ± 0.47 mm−1 and 4.89 ± 0.59 mm−1 at 150 min for 100 nm TiO2 NPs and 100 nm TiO2NPs/US, respectively. The results suggest that the optical properties of NL and LAT tissues are greatly influenced by TiO2 NPs and their combination with ultrasound.
Export citation and abstract BibTeX RIS
1. Introduction
As nanotechnology develops rapidly, nanoparticles have been playing an increasingly vital role and been utilized widely in drug delivery antibacterial materials, cosmetics, sunscreens, and electronics [1, 2]. Metal nanoparticles are being extensively used in various biomedical applications due to their small size to volume ratio and extensive thermal stability [3, 4]. Of special interest among metal nanoparticles are TiO2 NPs, with the property of absorption and reflection with ultraviolet (UV) lights, used extensively in a variety of cosmetics. In addition, as fine white powder, TiO2 NPs are also used widely as a pigment or additive for paints, paper, ceramics, pharmaceuticals, toothpaste, plastics, foods and other products [5–7]. The wide application of nanomaterials means that TiO2 NPs closely and frequently come into contact with humans. However, Oberdorster et al [8]. have reported that TiO2 NPs caused a greater pulmonary inflammatory response than TiO2 at the same mass burden, with greater amounts of TiO2 NPs entering the alveolar interstitium in the lungs. Such similar results were found by Sager et al [9]. Studies have revealed that TiO2 NPs are more toxic than fine particles [10–12]. Lee et al [10]. also revealed that rats suffered from lung tumors after 2 y of exposure to high concentrations of fine TiO2 particles. Li et al [13]. have shown that instilled TiO2 NPs could induce lung damage, and change the permeability of the alveolar–capillary barrier.
Lung cancer is the leading cause of cancer deaths in men and the second leading cause of cancer deaths in women, with approximately 1.6 million new cases of lung cancer diagnosed and 1.4 million deaths each year throughout the world [14–16]. Environmental exposures are non-negligible factors that may contribute to regional variations in lung cancer rates [14]. TiO2 NPs are substantially potential health risks to humans, because long-term human exposure and environmental release of TiO2 NPs may cause incidences of lung lesions [10]. Thus, it is very necessary to study the effect of TiO2 NPs penetrating and accumulating in the human body, especially in lung tissue, for which human long-term exposure to TiO2 NPs increases the unintended target absorption, and therefore, contributes to pulmonary physiological side effects [8–13].
Optical techniques based on light absorption and scattering by components of the medium are indispensable for objective visualization of nanoparticles accumulation in biotissues. Optical coherence tomography (OCT) is a noninvasive technique that is sensitive to changes of optical characteristics of biotissues, based on the principles of low-coherency interferometry, in which the light of the near-IR range (0.75–1.3 µm) is used [17–19]. The method enables investigation of the internal microstructure of biotissues at depths of up to 2 mm with the spatial resolution 10–15 µm without breaking their integrity. OCT has been used extensively in tissue studies [20–24] to measure permeation of different molecules and materials through various epithelial tissues, such as rabbit sclera [21], monkey skin [22], rabbit cornea [23], porcine aorta [22, 24], human skin [25] and human esophagus [26]. In addition, DR spectra is also a noninvasive method used widely in monitoring analyses concentrations in bulk tissue and investigating tissue optical parameters [27–31]. It is one of the simplest and most cost effective methods for understanding biological tissue characteristics. This technique involves detection and analysis of a portion of the incident light that undergoes multiple elastic scattering owing to inhomogeneities in the refractive index of the tissue. To have a further investigation of the interactions between nanoparticles and deeper depth of lung tissues, we also used ultrasound to change tissue status, which may improve the nanoparticles penetration into biological tissues. Ultrasound-induced cavitation, as a nondestructive physical method, has been shown to enhance analytes delivery in vitro and in vivo [32–35]. However, few studies have reported ultrasound-mediated nanoparticles, especially TiO2 NPs penetration into biotissues.
A growing number of experimental studies have focused on the study of toxicological information of TiO2 NPs within different organs [36, 37] and some studies focused on the research of TiO2 NPs accumulation in human or animal skin [38, 39]. Our group has reported the effect of nanoparticles accumulation on the optical properties of ex vivo human colon tissue [17]. However, there are few studies to investigate TiO2 NPs penetrating and accumulating into the normal and cancerous human lung tissues and thus induced changes in optical properties of the lung tissue. Herein, in order to assess the effects of TiO2 NPs on optical properties of human lung tissue for further understanding the interactions between TiO2 NPs and lung tissue, we monitored the continuous process of TiO2 NPs penetrating and accumulating in normal and cancerous lung tissues with OCT and DR spectra. And then we evaluated the effects of the different sizes of TiO2 NPs on optical properties of lung tissue and also changed tissue status by ultrasound to research the effects of different sized TiO2 NPs in combination with ultrasound on tissue optical properties.
2. Materials and methods
2.1. OCT System
Visualization of lung tissue filled with the suspension of the particles was implemented using a commercial spectral domain OCT (SD-OCT) system (Shenzhen MOPTIM Imaging Technique Co, Ltd, China) working at the central wavelength 830 ± 40 nm with an optical power of 5 mW, a maximum image depth of 1.6 mm, a signal-to-noise ratio of 120 dB and a length of scanned area 2, 3, 4 and 5 mm for choice. The axial and lateral resolution determined by the focal spot size of the probe beam, are 12 µm and 15 µm, respectively. 2D images are obtained through scanning the incident beam over the sample surface in the lateral direction and in-depth (A-scan) scanning by the interferometer. The acquisition time per OCT image is about 180 ms, corresponding to an A-scan frequency of 2000 Hz. A computer is used to control the OCT system with data acquisition software written in LabView 7.2D. OCT images obtained in the experiment were stored in the computer for further post-processing.
2.2. Diffuse reflectance and ultrasound system
The reflectance spectra of lung tissue were measured using a commercial optical fiber spectrometer (Ocean Optics, USA, model: USB 4000) in the spectral range 200 to 1100 nm. This spectrometer is equipped with a 3648-element Toshiba Linear CCD Array that gives a spectral resolution of 6 nm in conjunction with the 400 µm-diameter optical fiber light guide. The tungsten halogen light source (Ocean Optics, USA, model: LS-450) served as a source of light. The fiber-optic probe (Ocean Optics, USA, model: USB 4000) consisting of seven fibers with the internal diameter 400 µm and the numerical aperture 0.2 was used in the measurements. The central fiber served for collecting the diffuse reflected radiation, while the surrounding six fibers were used for the illumination of the sample. The spectra were calibrated against a DR standard of BaSO4 with a smooth surface. The probe was placed at the distance of 2 mm from the sample surface and registered the signal, averaged over the area of the radiation collection. All spectra measurements were recorded with the integration time set to 100 ms. The measurements were recorded and stored onto a computer for further post-processing.
A sonicator (DM-F608, Dimyth Beauty Equipment Manufacture, Guangzhou, China) with a frequency of about 1 MHz and an intensity of 0.8 W cm−2 was used for ultrasound application. The sonicator with this frequency is most commonly used in beauty treatment and transdermal drug delivery. The probe with a diameter of about 0.8 cm was applied. In order to avoid any thermal effects, a pulsed mode of ultrasound (500 ms pulses applied every second) was selected. During sonication, the ultrasound probe was immersed in the topical applied physiological saline with sufficient contact pressure.
2.3. TiO2 NPs preparation
In this experiment, we used two different sizes of commercially available high purity (99.8%) rutile TiO2 nanoparticles (Aladdin Chemistry Co, Ltd, Shanghai, China). The sizes of 60 nm and 100 nm TiO2 NPs were used, which are the primary particle sizes of food grade TiO2 and are also the main TiO2 NPs sizes existing in personal care products [40]. The transmission electron microscopy (TEM) images are shown in figure 1. A nanoparticles suspension was prepared by dissolving nanosize titanium dioxide powder in distilled water. The obtained suspension had the concentration of 1 mg ml−1. And then the suspension was dispersed in an ultrasonic bath (the power of 35 W and the frequency of 43 to 45 kHz) for 30–40 min for thorough mixing of the content both in the process of preparation and immediately before use.
Figure 1. Electron microphotographs of TiO2 nanoparticles: (a) 60 nm, (b) 100 nm.
Download figure:
Standard image High-resolution image2.4. Tissue preparation and experimental procedure
Surgically resected human lung adenocarcinoma tumor tissues were obtained from the First Affiliated Hospital of Sun Yat-Sen University, from where the study protocol was approved by the Ethics Committee and signed informed consents were obtained from all ten patients. Normal lung tissues were peeled off from the edges of tumor tissues. The fresh tissues were immediately rinsed in physiological saline to remove surface excess blood and stored in a refrigerator at −70 °C until in vitro measurement. All the samples of size 1.2 × 1.2 cm2 were accurately cut off from lung tissues in the frozen state. A total of eight groups were divided and are shown in table 1. Each group contains eight specimens, half of which were used for continuous OCT monitoring, and the other half were used for DR measurement.
Table 1. Groups and treatment in the experiments.
| Groups | Normal lung tissue | Groups | Lung adenocarcinoa tumor tissue |
|---|---|---|---|
| 1 | 60 nm TiO2 NPs | 5 | 60 nm TiO2 NPs |
| 2 | 60 nm TiO2 NPs/US | 6 | 60 nm TiO2 NPs/US |
| 3 | 100 nm TiO2 NPs | 7 | 100 nm TiO2 NPs |
| 4 | 100 nm TiO2 NPs/US | 8 | 100 nm TiO2 NPs/US |
Before each measurement, samples were defrosted in physiological saline at room temperature for 30 min. For OCT monitoring, the selected region of each sample was monitored for about 8 to 10 min by the OCT system to get a baseline before any application. Then, 25 µl drops of TiO2 nanoparticles suspensions were applied on the lung tissue surfaces. In the sonophoresis experiments, ultrasound was treated for 10 min at each sample before surface applying nanoparticles suspensions. The samples were continuously monitored for the next 240 min at 20 °C immediately after the application of the nanoparticles solutions. The DR spectra of samples were measured every 30 min for the duration of 240 min after administering the nanoparticles solutions, while the spectra obtained before the administration were used as a control. The suspensions of sample surfaces were wiped gently with a tampon wetted with saline prior to DR measurement. After each measurement, TiO2 nanoparticles suspensions were applied again on the tissue. During the procedure, the samples were placed in a Petri dish with a small volume of saline to prevent the change in the optical properties due to dehydration. Each sample was used only once.
2.5. Data processing
OCT imaging is based on the difference of backscattered light. The reflected light intensity depends on the tissue optical properties. To characterize the changes in optical properties of lung tissues during the penetration and accumulation of TiO2 nanoparticles, the attenuation coefficients of each sample were calculated from the 2D OCT image. For media with absorption as described by the single-scattering approximation, the light travels in a ballistic way and Beer's law can be applied to calculate the total OCT attenuation coefficient: μt = μa + μs, where μa is the absorption coefficient and μs is the scattering coefficient [41]. These are physical properties unique to the biological tissue, which play a vital role in the assessment of the tissue feature [41–43]. In this current OCT system case, the measured signal is defined as [42–44]:
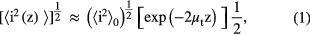
where the  is the photo detector heterodyne signal current received by the OCT system from the probing depth z and
is the photo detector heterodyne signal current received by the OCT system from the probing depth z and  is the mean square heterodyne signal. The result of the OCT study is the measurement of optical backscattering or reflectance
is the mean square heterodyne signal. The result of the OCT study is the measurement of optical backscattering or reflectance ![$R\left(z\right)\propto {{\left[\left\langle {{i}^{2}}\left(z\right)\right\rangle \right]}^{\frac{1}{2}}}$](https://content.cld.iop.org/journals/1555-6611/24/11/115606/revision1/lp501660ieqn003.gif) from a tissue versus axial ranging distance or depth, z. The reflectance depends on the optical properties of tissue, i.e. the total attenuation coefficient μt. Thus, combined with equation (1) and R (z) it follows that the reflected power can be approximately proportional to −μtz in exponential scale according to the single scattering model:
from a tissue versus axial ranging distance or depth, z. The reflectance depends on the optical properties of tissue, i.e. the total attenuation coefficient μt. Thus, combined with equation (1) and R (z) it follows that the reflected power can be approximately proportional to −μtz in exponential scale according to the single scattering model:
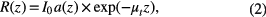
where I0 is the incident light intensity launched into the tissue sample and a(z) is the reflectivity of the tissue at depth z. a(z) is linked to the local refractive index and backscattering property of the tissue [45, 46]. However, the reflectivity a(z) is considered as weakly dependent on depth for a homogeneous tissue layer. Therefore, μt can be obtained theoretically from the reflectance measurements at two different depths: z1 and z2 [42–44].
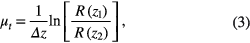
where  As noise is inevitable in the measurement, a final result should thus be obtained by the use of a best-fit exponential curve technique in order to improve the accuracy of the determined value of μt. An averaged optical intensity profile that represents the reflected light intensity distribution in depth is obtained by averaging the 2D image laterally over 1 mm, which is wide enough for speckle noise suppression.
As noise is inevitable in the measurement, a final result should thus be obtained by the use of a best-fit exponential curve technique in order to improve the accuracy of the determined value of μt. An averaged optical intensity profile that represents the reflected light intensity distribution in depth is obtained by averaging the 2D image laterally over 1 mm, which is wide enough for speckle noise suppression.
Quantitative data were obtained by averaging the linearized signal intensity across the lateral imaging range as a function of depth. A best-fit exponential curve covering the lung tissue in depth was applied to the averaged and normalized signal intensity data from which the corresponding 1 e−1 light penetration depth was derived [26, 47].
In the spectroscopic measurements, the changes of reflectance induced by nanoparticles solutions were quantitatively evaluated by calculating the increase in DR after 60, 150 and 210 min treatment. The increase in DR by the solutions at the time intervals of treatment was calculated as equation (4) [48, 49].
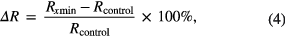
where Rcontrol and Rx min are the measured diffuse reflectance at 543 nm of each group of samples before and after x min treatment in different ways. The reason for selecting 543 nm to assess reflectance is that it represents visible wavelength with skin oxyhemoglobin absorption [50–52].
2.6. Statistical analysis
The data from all samples were presented as means ± SD and analyzed by an SPSS 20.0 software paired-test. The p < 0.05 value indicated significant difference.
3. Results and discussion
In this research, the main purpose of this pilot study was to evaluate the effects of different sized TiO2 NPs penetrating and accumulating in normal and cancerous human lung tissues on their optical properties, and additionally, to research the influence of different sized TiO2 NPs in combination with ultrasound on lung tissue optical properties. Figures 2 and 3 illustrate the dynamic changes of diffuse reflectance, as a function of time elapsed after the NL and LAT tissue samples were topically treated with 60 or 100 nm TiO2 NPs alone and 60 or 100 nm TiO2 NPs in combination with ultrasound, respectively. The absorption bands of blood hemoglobin at the wavelengths 416 (Soret band), 543 and 578 nm are very pronounced [51, 52]. The increase in the intensity of radiation reflected from the sample was observed, which is related to the substantial increase of the scattering coefficient of the tissue, promoted by the nanoparticles located in the lung tissues. In order to quantitatively evaluate the changes of reflectance induced by solutions, we calculated the enhancement of diffuse reflectance after 60, 150 and 210 min nanoparticles solutions treatment at 543 nm. The results for reflectance are illustrated in figure 4. From figure 4, it is obvious to see that the enhancement of DR for LAT tissue is markedly larger than that for NL tissue when under the same treatment. The main reason for such phenomenon may be that LAT tissues are easier to penetrate compared with NL tissues, thus, more nanoparticles would spread into the tissue in LAT tissue and induce higher enhancement of diffuse reflectance. The mixed treatments, including 60 nm TiO2 NPs/US and 100 nm TiO2 NPs/US, are more effective in enhancement of DR than any single solutions. This phenomenon could be induced by the cavitation effect of ultrasound, which has been demonstrated in TDD experiments [34, 35]. This effect not only makes biological tissues more permeable but also promotes more nanoparticles penetrating into tissues. In addition, the DR of 100 nm TiO2 NPs treatment groups obtained much more visible enhancement than 60 nm TiO2 NPs treatment groups under the same condition. This may be caused by the fact that 100 nm TiO2 NPs have a larger backscattering and thus generate bigger enhancement of DR. It is noticed that the larger increases in diffuse reflectance after 210 min for 60 nm TiO2 NPs and 60 nm TiO2 NPs/US were observed in NL tissue when compared with those at 150 min, whereas there were only slight increases for 100 nm TiO2 NPs and 100 nm TiO2 NPs/US. This phenomenon may be caused by the effect of different sizes of TiO2 NPs. The same trend, however, not pronouncedly, was observed in LAT tissue. As a result, it can be assumed that 60 nm TiO2 NPs may penetrate faster and reach the maximum amount of penetration earlier than 100 nm TiO2 NPs.
Figure 2. Measured diffuse reflectance spectra over 400–1100 nm for human NL tissue in vitro before and after the different applications at 60, 150 and 210 min: (a) the solid line represents 60 nm TiO2 NPs treatment, the dashed line represents 60 nm TiO2 NPs/US treatment; (b) the solid line represents 100 nm TiO2 NPs treatment, the dashed line represents 100 nm TiO2 NPs/US treatment.
Download figure:
Standard image High-resolution imageFigure 3. Measured diffuse reflectance spectra over 400–1100 nm for human LAT tissue in vitro before and after the different applications at 60, 150 and 210 min: (a) the solid line represents 60 nm TiO2 NPs treatment, the dashed line represents 60 nm TiO2 NPs/US treatment; (b) the solid line represents 100 nm TiO2 NPs treatment, the dashed line represents 100 nm TiO2 NPs/US treatment.
Download figure:
Standard image High-resolution imageFigure 4. Enhancement of diffuse reflectance at 543 nm for human lung tissues after the different applications of TiO2 NP solutions at 60, 150 and 210 min: (a) NL tissue; (b) LAT tissue.
Download figure:
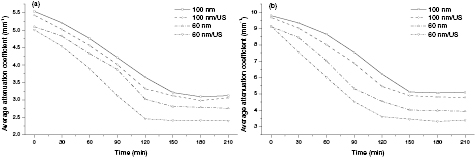
Standard image High-resolution imageFor further study of the effects of TiO2 NPs penetrating and accumulating in normal and cancerous human lung tissues on their optical properties under different treatments, the attenuation coefficients of each group were calculated from the data of the best-fit exponential curve corresponding to the averaged intensity profiles. The selected region is from the depth of 148 µm to the depth of 308 µm, where the OCT signal distribution is relatively smooth. Figures 5(a) and (b) present the changes in the attenuation coefficients of NL and LAT treated with 60 nm TiO2 NPs, 60 nm TiO2 NPs/US, 100 nm TiO2 NPs and 100 nm TiO2 NPs/US, respectively. From figure 5(a), we can see the attenuation coefficients of NL tissue are about 5.10 ± 0.26 mm−1 for 60 nm TiO2 NPs treatment and 5.01 ± 0.36 mm−1 for 60 nm TiO2 NPs/US treatment at 0 min and then it changed to be 3.12 ± 0.43 mm−1 for 60 nm TiO2 NPs and 2.15 ± 0.54 mm−1 for 60 nm TiO2NPs/US at 120 min when the penetration process reached the stable state. However, the starting values of average attenuation coefficients of NL tissue are 5.54 ± 0.46 mm−1 and 5.43 ± 0.64 mm−1 for the treatment of 100 nm TiO2 NPs and 100 nm TiO2NPs/US, respectively, while it took about 150 min to reach the stable state and the values became 3.24 ± 0.73 mm−1 and 2.69 ± 0.34 mm−1 for 100 nm TiO2 NPs and 100 nm TiO2NPs/US, respectively. By comparing these data, it can be concluded that 60 nm TiO2 NPs may penetrate faster and reach the maximum amount of penetration earlier than 100 nm TiO2 with the same condition. This is well consistent with the previous results of DR. And it is obvious that the attenuation coefficients in the same kind of lung tissues are almost equal at the beginning no matter whether they are treated with ultrasound or not. However, a significant difference in the attenuation coefficients occurs between the groups treated with ultrasound and those without during the TiO2 NPs penetration process and also between the groups with 60 nm TiO2 NPs and 100 nm TiO2 NPs. The attenuation coefficients were reduced by about 38% and 34% in NL tissue after 120 min treatment of 60 nm TiO2 NPs and 100 nm TiO2 NPs, respectively, while with the treatment of 60 or 100 nm TiO2 NPs/US, the attenuation coefficients were decreased by approximately 57% and 39%, respectively. Thus, we can conclude that the penetration process of TiO2 NPs solutions with both sizes has been dramatically accelerated by the sonophoresis. However, there are much more pronounced increase of 60 nm TiO2 NPs penetration in lung tissue induced by ultrasound than the penetration of 100 nm TiO2 NPs in the same type of lung tissue. In addition, the attenuation coefficients of 100 nm TiO2 NPs treatment groups are apparently larger than 60 nm TiO2 NPs treatment groups in the same condition. This may be caused by the fact that 100 nm TiO2 NPs have a larger backscattering compared with 60 nm TiO2 NPs, and thus generate a more substantial increase of the scattering coefficient of the tissue. This is well consistent with the results of previous figures 2 and 3. The same results were observed in LTA tissue shown in figure 5(b). The average attenuation coefficient of LAT tissue changes from 9.12 ± 0.54 mm−1 to 4.54 ± 0.39 mm−1 and 3.61 ± 0.38 mm−1 at 120 min for 60 nm TiO2 NPs and 60 nm TiO2NPs/US treatment, respectively, and from 9.79 ± 0.32 mm−1 to 5.12 ± 0.47 mm−1 and 4.89 ± 0.59 mm−1 at 150 min for 100 nm TiO2 NPs and 100 nm TiO2NPs/US, respectively. The results indicate that average attenuation coefficient of LAT tissue was significantly larger than that of NL tissue with the same treatment. It is likely related to the tumor tissue having stronger scattering than normal tissue due to larger nuclei; thus, the higher nuclear-to-cytoplasmic ratio in tumor cells, the higher regional tumor cell density of the tumor tissues [53, 54].
Figure 5. Average attenuation coefficients of human NL and LAT tissue after the different applications of TiO2 NP solutions: (a) NL sample group; (b) LAT sample group.
Download figure:
Standard image High-resolution imageA best-fit exponential curve covering of the NL and LAT tissues in depth were applied to the averaged and normalized signal intensity data from which the corresponding 1 e−1 light penetration depth was derived [26, 47]. Figures 6(a)–(d) show OCT depth intensity profiles with corresponding exponential best-fit curves of NL and LAT tissues before and after 150 min application of 60 nm TiO2 NPs, 60 nm TiO2 NPs/US, 100 nm TiO2 and 100 nm TiO2 NPs/US, respectively. From figures 6(a) and (b), the relative 1 e−1 light penetration depth values were calculated, which were found to be 0.37 ± 0.02 mm for the native NL tissue and 0.49 ± 0.02, 0.58 ± 0.03, 0.52 ± 0.05 and 0.61 ± 0.03 mm at 150 min for the treatments of 60 nm TiO2 NPs, 60 nm TiO2 NPs/US, 100 nm TiO2 NPs and 100 nm TiO2 NPs/US from NL tissues, respectively. The relative 1 e−1 light penetration depth value of LAT tissue is 0.31 ± 0.03 mm at the beginning, it changes to be 0.38 ± 0.02, 0.48 ± 0.04, 0.41 ± 0.05 and 0.45 ± 0.02 mm at 150 min for the treatments of 60 nm TiO2 NPs, 60 nm TiO2 NPs/US, 100 nm TiO2 NPs and 100 nm TiO2 NPs/US, respectively. From these data, we can find that the relative 1 e−1 light penetration depth for the NL tissues is relatively higher than that for LAT tissues with the same condition. It may be also induced by the difference in structure and morphology between the two different types of tissue [53, 54]. Meanwhile, it can be seen that there was only a small enhancement of 1 e−1 light penetration depth both in NL and LAT tissues with 60 nm TiO2 NPs treatment alone, whereas the 1 e−1 light penetration was greatly enhanced after 150 min 60 nm TiO2 NPs/US exposure both in NL and LAT tissues. For the treatment of 100 nm TiO2 NPs, it generated larger enhancement of 1 e−1 light penetration depth than 60 nm TiO2 NPs with the same condition, however, there was only a small enhancement with 100 nm TiO2 NPs/US compared with 100 nm TiO2 NPs alone at 150 min. The results show again that 100 nm TiO2 NPs have a larger backscattering compared with 60 nm TiO2 NPs and the synergistic effect of 60 nm TiO2 and sonophoresis on lung tissue optical properties is more effective than the effect of 100 nm TiO2 NPs in combination with sonophoresis on lung tissue optical properties. It is also well consistent with previous results of DR and attenuation coefficient.
Figure 6. Corresponding OCT intensity profile as a function of depth for human NL and LAT lung tissue before and after different applications of TiO2 NP solutions at 150 min: (a) and (b) NL tissue; (c) and (d) LAT tissue.
Download figure:
Standard image High-resolution image4. Conclusions
In this study, we have demonstrated the in vitro study of different sized TiO2 NPs penetration and accumulation in healthy and cancerous lung tissues by the methods of continuous OCT monitoring and DR measurement. Meanwhile, we also have assessed the effects of different sized TiO2 NPs in combination with ultrasound on lung tissue optical properties. By calculating and analyzing the values of enhancement of DR, average attenuation coefficient and the relative 1 e−1 light penetration depth, we can find that the penetration process of TiO2 NPs with both sizes (60 nm and 100 nm) in LAT tissue is faster than that in NL tissue and 60 nm TiO2 NPs penetrate and accumulate faster than 100 nm TiO2 NPs in the same type of lung tissue. In addition, the results demonstrate that sonophoresis can accelerate the penetration process of TiO2 NPs of both sizes in the lung tissues, however, the synergistic effect of 60 nm TiO2 and sonophoresis on optical properties of lung tissue is more effective than the effect of 100 nm TiO2 in combination with sonophoresis on lung tissue optical properties. Thus, results of our studies show that OCT and DR provides an ideal tool to perform qualitative and quantitative analyses of TiO2 NPs penetrating and accumulating in lung tissue to reveal the localization of nanoparticles within tissues and to investigate the changes in optical properties of biotissue induced by nanoparticles and its combination with US. The sized effects of nanoparticles combined with ultrasound on tissue optical properties need to be further explored.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 60778047, 61335011, 61275187 and 81071790), Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant Nos. 20114407110001 and 200805740003), the Science and Technology Innovation Project of the Education Department of Guangdong Province of China, the Natural Science Foundation of Guangdong Province of China (Grant Nos. 06025080 and 9251063101000009), Technique invention program of Guangdong Province (2013KJCX0052), the Science and Technology Project of Guangdong Province (2012A080203008) and Key Laboratory of Optoelectronic Science and Technology for Medicine (Fujian Normal University), Ministry of Education, China (Grant No. JYG1202).